Improving the Stability and Catalyst Lifetime of the Halogenase RebH By Directed Evolution.
Poor, C.B., Andorfer, M.C., Lewis, J.C.(2014) Chembiochem 15: 1286-1289
- PubMed: 24849696
- DOI: https://doi.org/10.1002/cbic.201300780
- Primary Citation of Related Structures:
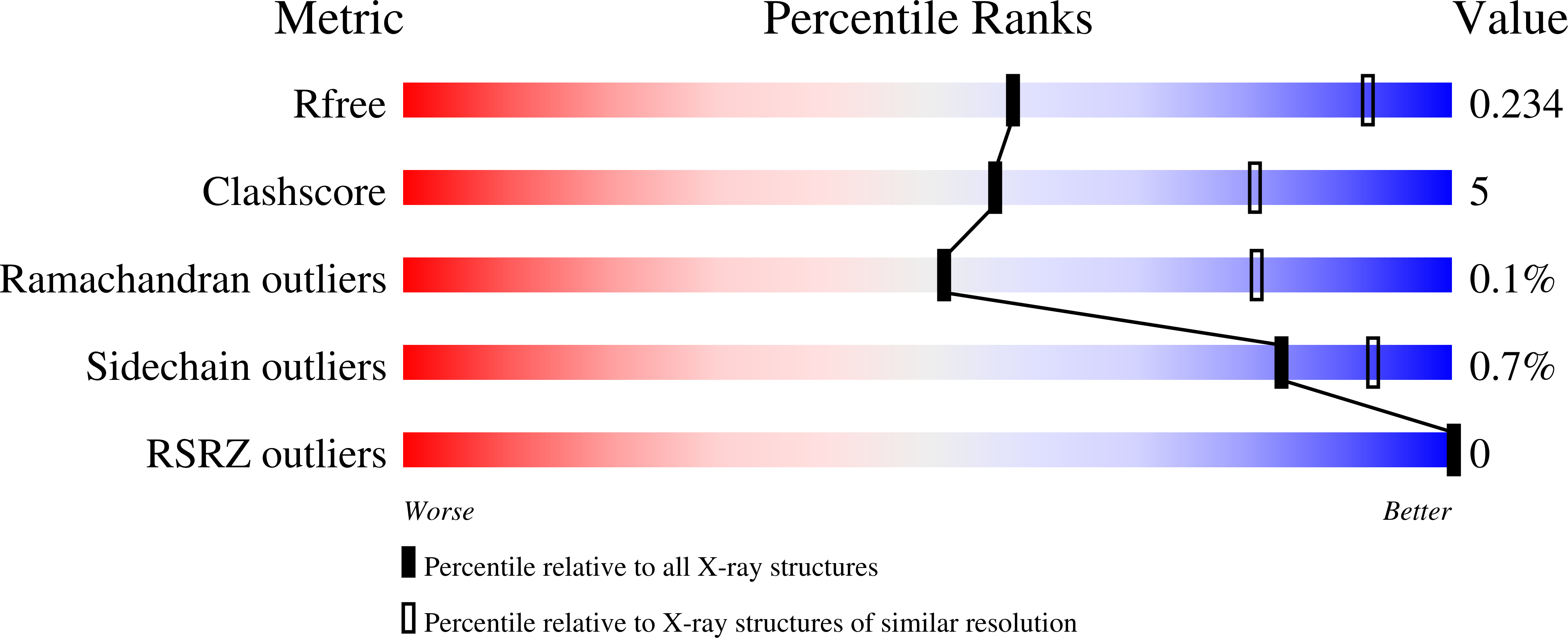
4LU6 - PubMed Abstract:
We previously reported that the halogenase RebH catalyzes selective halogenation of several heterocycles and carbocycles, but product yields were limited by enzyme instability. Here, we use directed evolution to engineer an RebH variant, 3-LR, with a Topt over 5 °C higher than that of wild-type, and 3-LSR, with a Tm 18 °C higher than that of wild-type. These enzymes provided significantly improved conversion (up to fourfold) for halogenation of tryptophan and several non-natural substrates. This initial evolution of RebH not only provides improved enzymes for immediate synthetic applications, but also establishes a robust protocol for further halogenase evolution.
Organizational Affiliation:
Department of Chemistry, University of Chicago, 5735 S. Ellis Ave., Chicago, IL 60637 (USA).