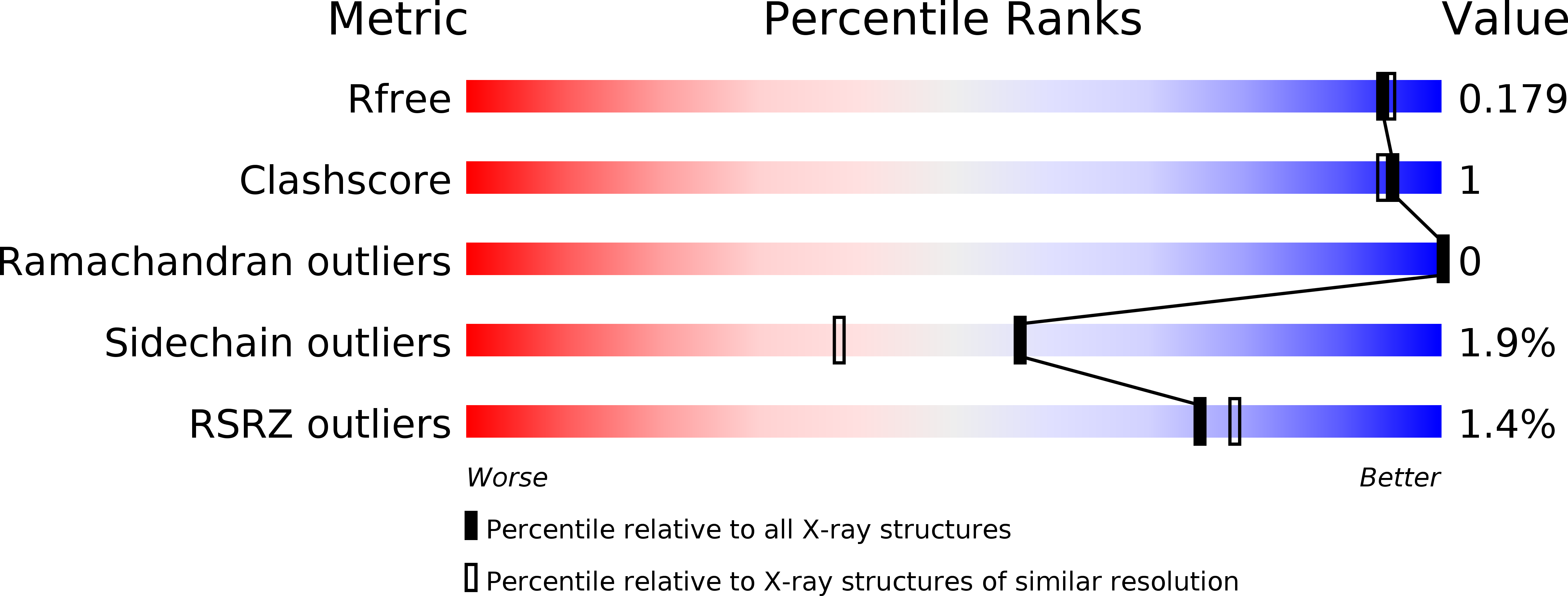
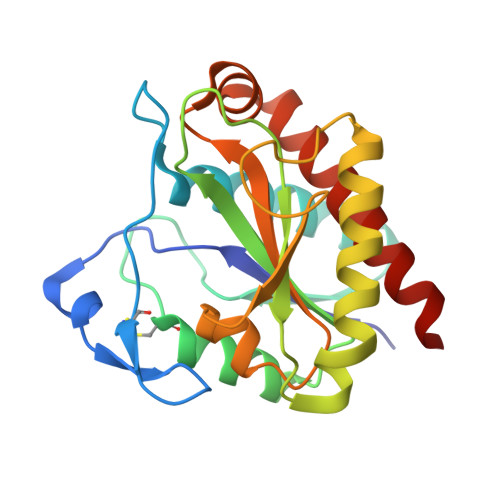
The structure of Rv3717 reveals a novel amidase from Mycobacterium tuberculosis.
Kumar, A., Kumar, S., Kumar, D., Mishra, A., Dewangan, R.P., Shrivastava, P., Ramachandran, S., Taneja, B.(2013) Acta Crystallogr D Biol Crystallogr 69: 2543-2554
- PubMed: 24311595
- DOI: https://doi.org/10.1107/S0907444913026371
- Primary Citation of Related Structures:
4LQ6 - PubMed Abstract:
Bacterial N-acetylmuramoyl-L-alanine amidases are cell-wall hydrolases that hydrolyze the bond between N-acetylmuramic acid and L-alanine in cell-wall glycopeptides. Rv3717 of Mycobacterium tuberculosis has been identified as a unique autolysin that lacks a cell-wall-binding domain (CBD) and its structure has been determined to 1.7 Å resolution by the Pt-SAD phasing method. Rv3717 possesses an α/β-fold and is a zinc-dependent hydrolase. The structure reveals a short flexible hairpin turn that partially occludes the active site and may be involved in autoregulation. This type of autoregulation of activity of PG hydrolases has been observed in Bartonella henselae amidase (AmiB) and may be a general mechanism used by some of the redundant amidases to regulate cell-wall hydrolase activity in bacteria. Rv3717 utilizes its net positive charge for substrate binding and exhibits activity towards a broad spectrum of substrate cell walls. The enzymatic activity of Rv3717 was confirmed by isolation and identification of its enzymatic products by LC/MS. These studies indicate that Rv3717, an N-acetylmuramoyl-L-alanine amidase from M. tuberculosis, represents a new family of lytic amidases that do not have a separate CBD and are regulated conformationally.
Organizational Affiliation:
Structural Biology Unit, CSIR-IGIB, South Campus, Mathura Road, New Delhi 110 025, India.