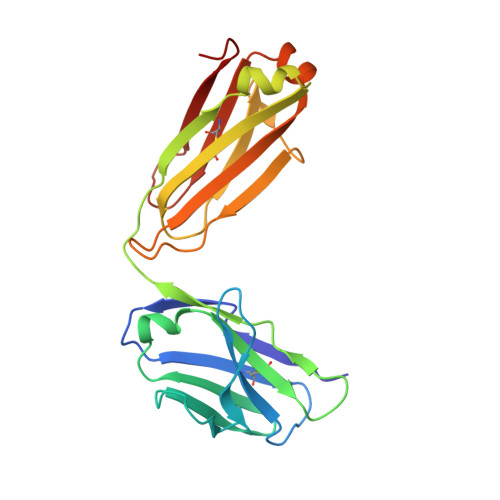
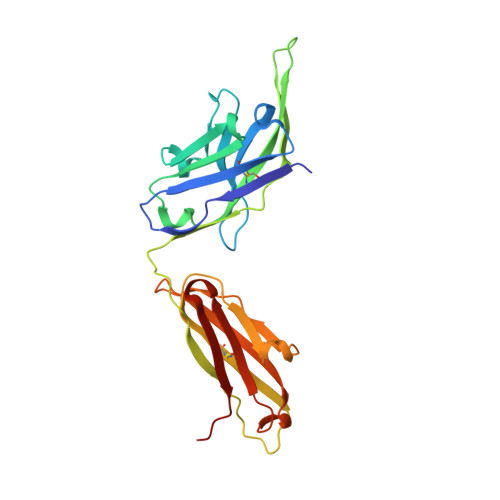
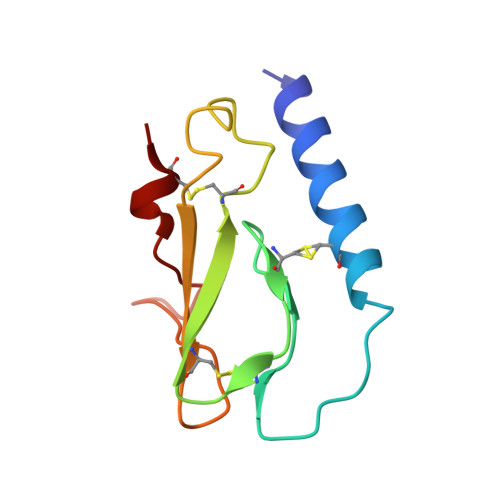
Inhibitory mechanism of an allosteric antibody targeting the glucagon receptor.
Mukund, S., Shang, Y., Clarke, H.J., Madjidi, A., Corn, J.E., Kates, L., Kolumam, G., Chiang, V., Luis, E., Murray, J., Zhang, Y., Hotzel, I., Koth, C.M., Allan, B.B.(2013) J Biol Chem 288: 36168-36178
- PubMed: 24189067
- DOI: https://doi.org/10.1074/jbc.M113.496984
- Primary Citation of Related Structures:
4LEX, 4LF3 - PubMed Abstract:
Elevated glucagon levels and increased hepatic glucagon receptor (GCGR) signaling contribute to hyperglycemia in type 2 diabetes. We have identified a monoclonal antibody that inhibits GCGR, a class B G-protein coupled receptor (GPCR), through a unique allosteric mechanism. Receptor inhibition is mediated by the binding of this antibody to two distinct sites that lie outside of the glucagon binding cleft. One site consists of a patch of residues that are surface-exposed on the face of the extracellular domain (ECD) opposite the ligand-binding cleft, whereas the second binding site consists of residues in the αA helix of the ECD. A docking model suggests that the antibody does not occlude the ligand-binding cleft. We solved the crystal structure of GCGR ECD containing a naturally occurring G40S mutation and found a shift in the register of the αA helix that prevents antibody binding. We also found that alterations in the αA helix impact the normal function of GCGR. We present a model for the allosteric inhibition of GCGR by a monoclonal antibody that may form the basis for the development of allosteric modulators for the treatment of diabetes and other class B GPCR-related diseases.
Organizational Affiliation:
From the Departments of Structural Biology.