Characterization of a nitrite reductase involved in nitrifier denitrification.
Lawton, T.J., Bowen, K.E., Sayavedra-Soto, L.A., Arp, D.J., Rosenzweig, A.C.(2013) J Biol Chem 288: 25575-25583
- PubMed: 23857587
- DOI: https://doi.org/10.1074/jbc.M113.484543
- Primary Citation of Related Structures:
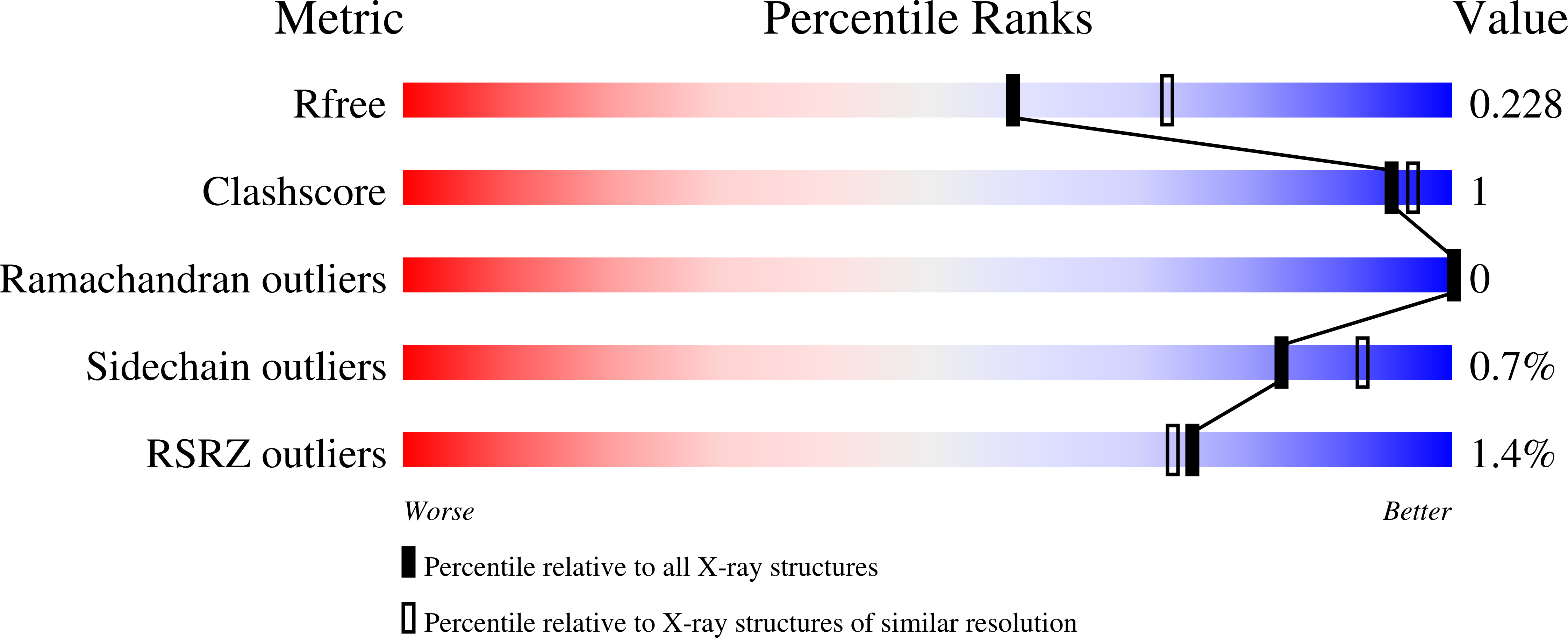
4KNS, 4KNT, 4KNU - PubMed Abstract:
Nitrifier denitrification is the conversion of nitrite to nitrous oxide by ammonia-oxidizing organisms. This process, which is distinct from denitrification, is active under aerobic conditions in the model nitrifier Nitrosomonas europaea. The central enzyme of the nitrifier dentrification pathway is a copper nitrite reductase (CuNIR). To understand how a CuNIR, typically inactivated by oxygen, functions in this pathway, the enzyme isolated directly from N. europaea (NeNIR) was biochemically and structurally characterized. NeNIR reduces nitrite at a similar rate to other CuNIRs but appears to be oxygen tolerant. Crystal structures of oxidized and reduced NeNIR reveal a substrate channel to the active site that is much more restricted than channels in typical CuNIRs. In addition, there is a second fully hydrated channel leading to the active site that likely acts a water exit pathway. The structure is minimally affected by changes in pH. Taken together, these findings provide insight into the molecular basis for NeNIR oxygen tolerance.
Organizational Affiliation:
From the Departments of Molecular Biosciences and of Chemistry, Northwestern University, Evanston, Illinois 60208 and.