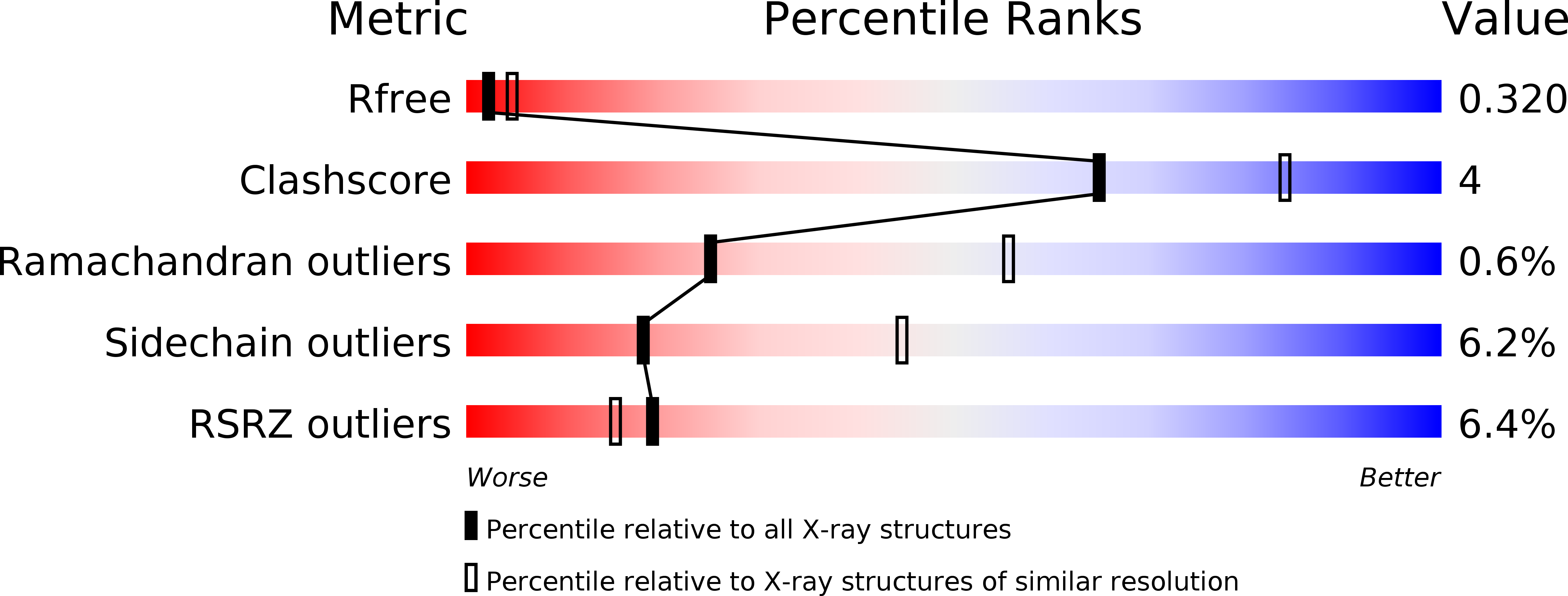
Reconstitution and characterization of eukaryotic N6-threonylcarbamoylation of tRNA using a minimal enzyme system.
Wan, L.C., Mao, D.Y., Neculai, D., Strecker, J., Chiovitti, D., Kurinov, I., Poda, G., Thevakumaran, N., Yuan, F., Szilard, R.K., Lissina, E., Nislow, C., Caudy, A.A., Durocher, D., Sicheri, F.(2013) Nucleic Acids Res 41: 6332-6346
- PubMed: 23620299
- DOI: https://doi.org/10.1093/nar/gkt322
- Primary Citation of Related Structures:
4K25 - PubMed Abstract:
The universally conserved Kae1/Qri7/YgjD and Sua5/YrdC protein families have been implicated in growth, telomere homeostasis, transcription and the N6-threonylcarbamoylation (t(6)A) of tRNA, an essential modification required for translational fidelity by the ribosome. In bacteria, YgjD orthologues operate in concert with the bacterial-specific proteins YeaZ and YjeE, whereas in archaeal and eukaryotic systems, Kae1 operates as part of a larger macromolecular assembly called KEOPS with Bud32, Cgi121, Gon7 and Pcc1 subunits. Qri7 orthologues function in the mitochondria and may represent the most primitive member of the Kae1/Qri7/YgjD protein family. In accordance with previous findings, we confirm that Qri7 complements Kae1 function and uncover that Qri7 complements the function of all KEOPS subunits in growth, t(6)A biosynthesis and, to a partial degree, telomere maintenance. These observations suggest that Kae1 provides a core essential function that other subunits within KEOPS have evolved to support. Consistent with this inference, Qri7 alone is sufficient for t(6)A biosynthesis with Sua5 in vitro. In addition, the 2.9 Å crystal structure of Qri7 reveals a simple homodimer arrangement that is supplanted by the heterodimerization of YgjD with YeaZ in bacteria and heterodimerization of Kae1 with Pcc1 in KEOPS. The partial complementation of telomere maintenance by Qri7 hints that KEOPS has evolved novel functions in higher organisms.
Organizational Affiliation:
Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON M5G 1X5, Canada.