Chemoenzymatic synthesis of new 2,4-syn-functionalized (S)-glutamate analogues and structure-activity relationship studies at ionotropic glutamate receptors and excitatory amino acid transporters.
Assaf, Z., Larsen, A.P., Venskutonyte, R., Han, L., Abrahamsen, B., Nielsen, B., Gajhede, M., Kastrup, J.S., Jensen, A.A., Pickering, D.S., Frydenvang, K., Gefflaut, T., Bunch, L.(2013) J Med Chem 56: 1614-1628
- PubMed: 23414088
- DOI: https://doi.org/10.1021/jm301433m
- Primary Citation of Related Structures:
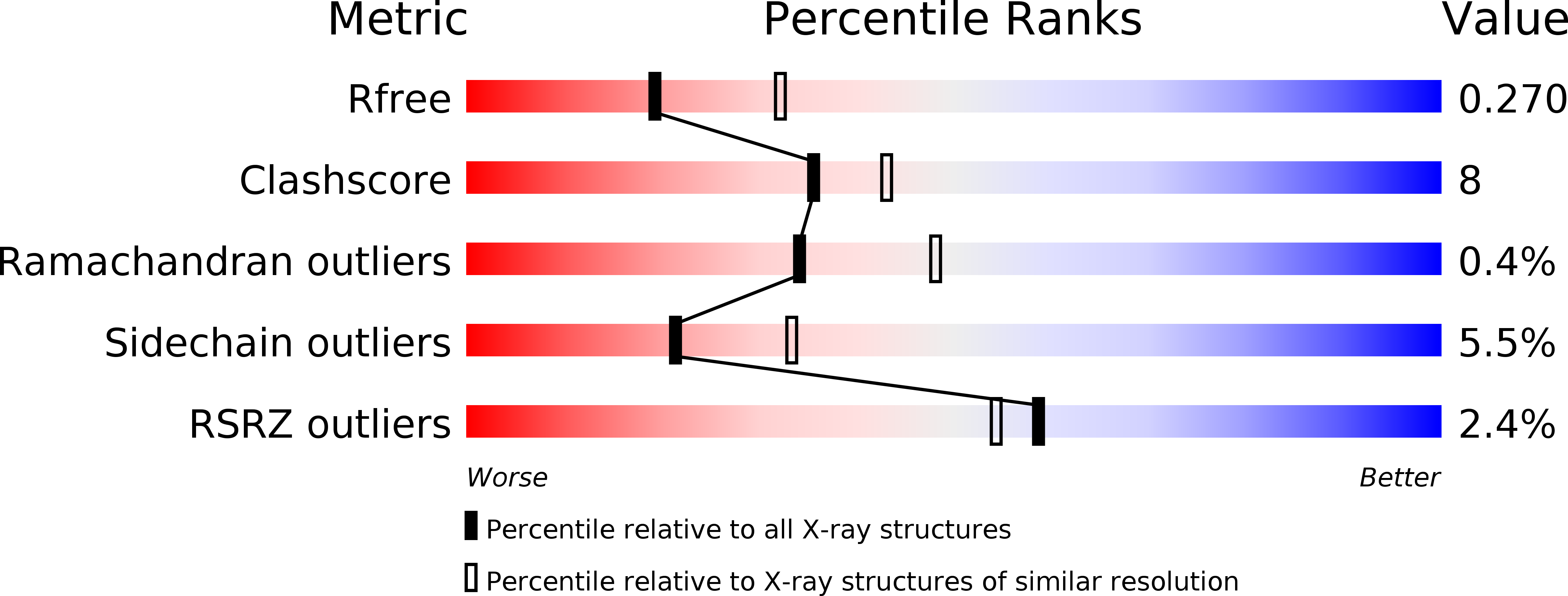
4IGR, 4IGT - PubMed Abstract:
In the mammalian central nervous system, (S)-glutamate (Glu) is released from the presynaptic neuron where it activates a plethora of pre- and postsynaptic Glu receptors. The fast acting ionotropic Glu receptors (iGluRs) are ligand gated ion channels and are believed to be involved in a vast number of neurological functions such as memory and learning, synaptic plasticity, and motor function. The synthesis of 14 enantiopure 2,4-syn-Glu analogues 2b-p is accessed by a short and efficient chemoenzymatic approach starting from readily available cyclohexanone 3. Pharmacological characterization at the iGluRs and EAAT1-3 subtypes revealed analogue 2i as a selective GluK1 ligand with low nanomolar affinity. Two X-ray crystal structures of the key analogue 2i in the ligand-binding domain (LBD) of GluA2 and GluK3 were determined. Partial domain closure was seen in the GluA2-LBD complex with 2i comparable to that induced by kainate. In contrast, full domain closure was observed in the GluK3-LBD complex with 2i, similar to that of GluK3-LBD with glutamate bound.
Organizational Affiliation:
Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, 2100 Copenhagen Ø, Denmark.