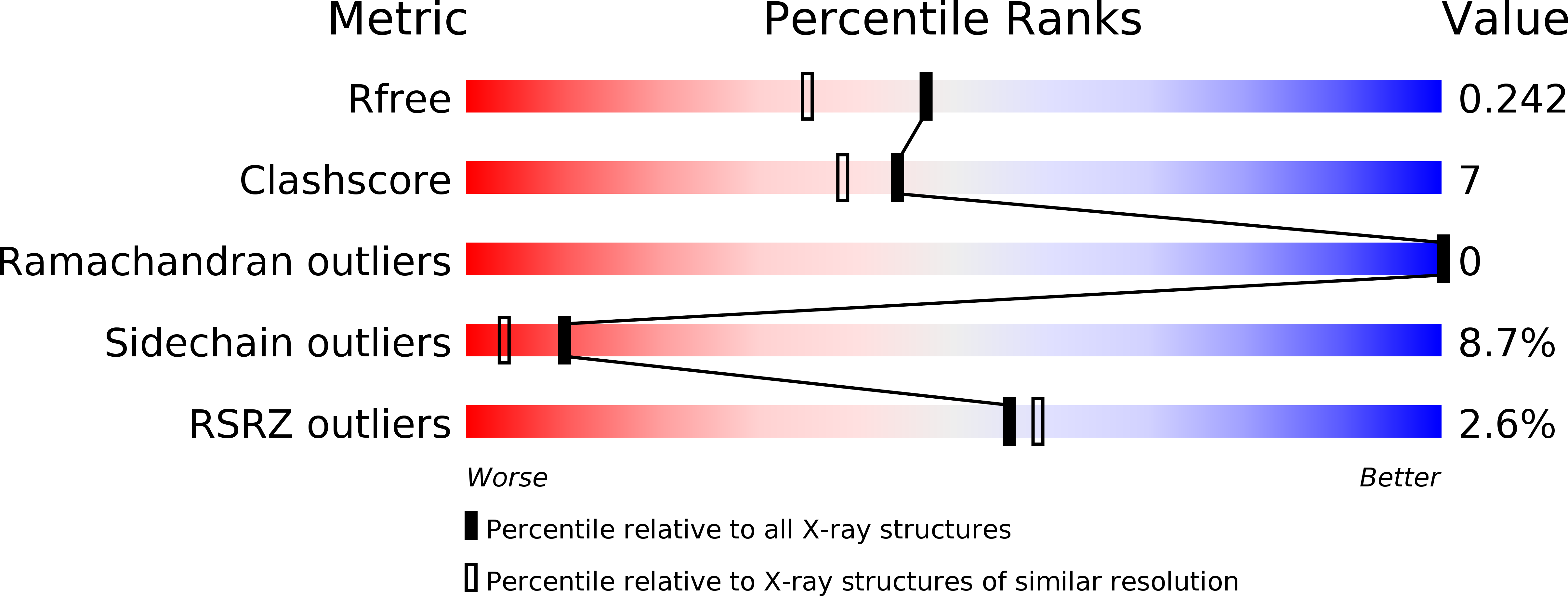
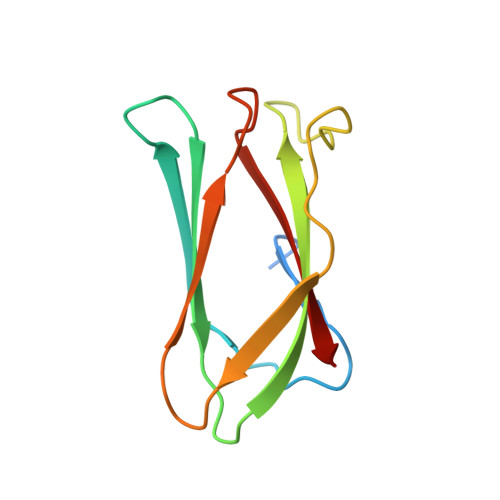
Crystal structure of the Campylobacter jejuni Cj0090 protein reveals a novel variant of the immunoglobulin fold among bacterial lipoproteins.
Paek, S., Kawai, F., Choi, K.J., Yeo, H.J.(2012) Proteins 80: 2804-2809
- PubMed: 22987763
- DOI: https://doi.org/10.1002/prot.24182
- Primary Citation of Related Structures:
4GIO - PubMed Abstract:
Bacterial lipoproteins play an important role in bacterial pathogenesis and physiology. The genome of Campylobacter jejuni, a major foodborn pathogen, is predicted to contain over 20 lipoproteins. However, the functions of the majority of C. jejuni lipoproteins remain unknown. The Cj0090 protein is encoded by a lipoprotein operon composed of cj0089, cj0090, and cj0091. Here, we report the crystal structure of Cj0090 at 1.9 Å resolution, revealing a novel variant of the immunoglobulin fold with β-sandwich architecture. The structure suggests that Cj0090 may be involved in protein-protein interactions, consistent with a possible role for bacterial lipoproteins.
Organizational Affiliation:
Department of Biology and Biochemistry, University of Houston, TX 77204, USA.