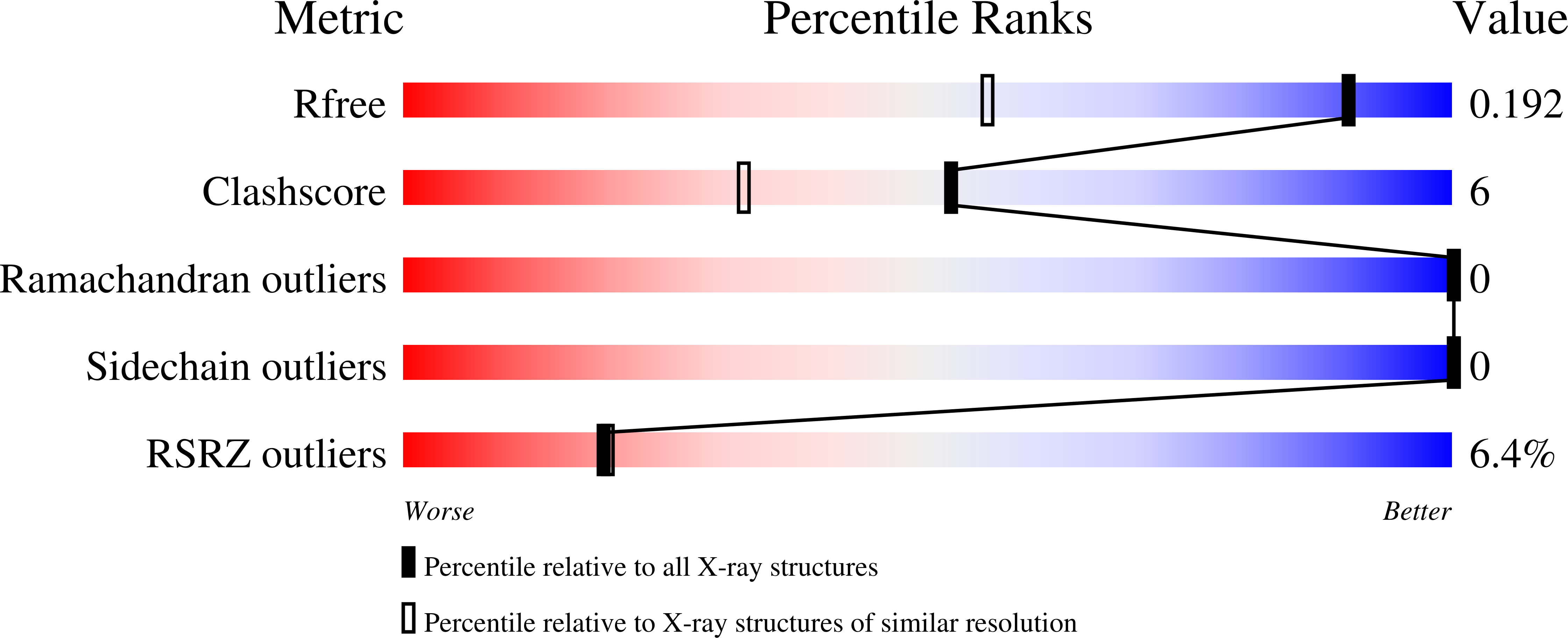
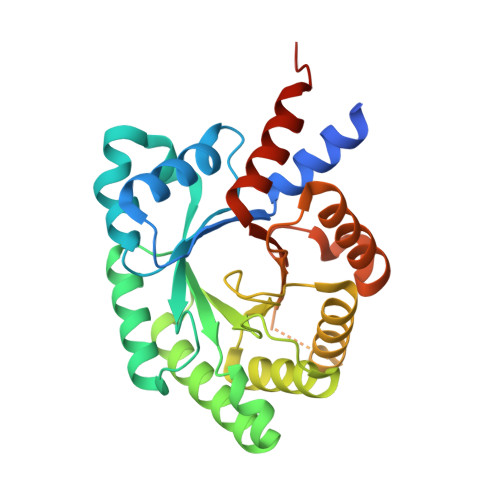
Structural and Functional Divergence of the Aldolase Fold in Toxoplasma gondii.
Tonkin, M.L., Halavaty, A.S., Ramaswamy, R., Ruan, J., Igarashi, M., Ngo, H.M., Boulanger, M.J.(2015) J Mol Biol 427: 840-852
- PubMed: 25284756
- DOI: https://doi.org/10.1016/j.jmb.2014.09.019
- Primary Citation of Related Structures:
3QYQ, 4D2J, 4EIV - PubMed Abstract:
Parasites of the phylum Apicomplexa are highly successful pathogens of humans and animals worldwide. As obligate intracellular parasites, they have significant energy requirements for invasion and gliding motility that are supplied by various metabolic pathways. Aldolases have emerged as key enzymes involved in these pathways, and all apicomplexans express one or both of fructose 1,6-bisphosphate (F16BP) aldolase and 2-deoxyribose 5-phosphate (dR5P) aldolase (DERA). Intriguingly, Toxoplasma gondii, a highly successful apicomplexan parasite, expresses F16BP aldolase (TgALD1), d5RP aldolase (TgDERA), and a divergent dR5P aldolase-like protein (TgDPA) exclusively in the latent bradyzoite stage. While the importance of TgALD1 in glycolysis is well established and TgDERA is also likely to be involved in parasite metabolism, the detailed function of TgDPA remains elusive. To gain mechanistic insight into the function of different T. gondii aldolases, we first determined the crystal structures of TgALD1 and TgDPA. Structural analysis revealed that both aldolases adopt a TIM barrel fold accessorized with divergent secondary structure elements. Structural comparison of TgALD1 and TgDPA with members of their respective enzyme families revealed that, while the active-site residues are conserved in TgALD1, key catalytic residues are absent in TgDPA. Consistent with this observation, biochemical assays showed that, while TgALD1 was active on F16BP, TgDPA was inactive on dR5P. Intriguingly, both aldolases are competent to bind polymerized actin in vitro. Altogether, structural and biochemical analyses of T. gondii aldolase and aldolase-like proteins reveal diverse functionalization of the classic TIM barrel aldolase fold.
Organizational Affiliation:
Biochemistry and Microbiology, University of Victoria, PO Box 3055 STN CSC, Victoria, BC, Canada V8W 3P6.