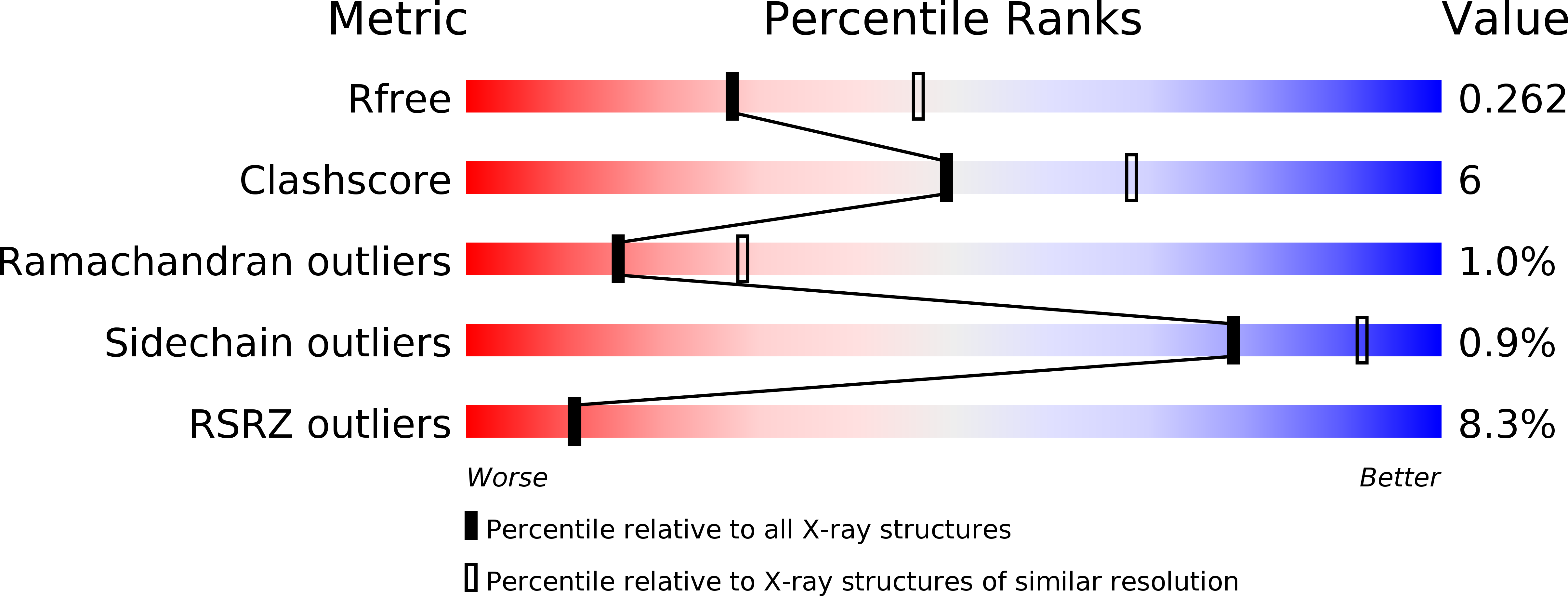
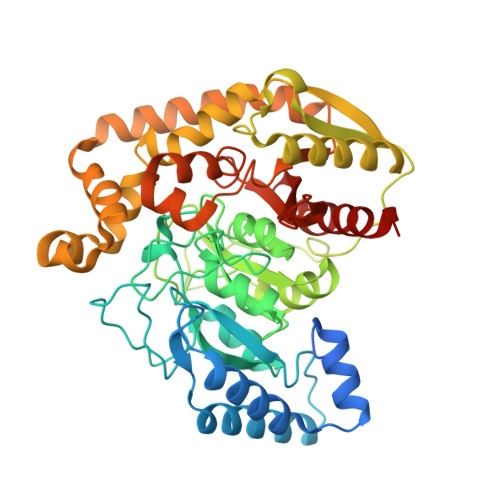
X-Ray Structure of the Amidase Domain of Atzf, the Allophanate Hydrolase from the Cyanuric Acid-Mineralizing Multienzyme Complex.
Balotra, S., Newman, J., Cowieson, N.P., French, N.G., Campbell, P.M., Briggs, L.J., Warden, A.C., Easton, C.J., Peat, T.S., Scott, C.(2015) Appl Environ Microbiol 81: 470
- PubMed: 25362066
- DOI: https://doi.org/10.1128/AEM.02783-14
- Primary Citation of Related Structures:
4CP8 - PubMed Abstract:
The activity of the allophanate hydrolase from Pseudomonas sp. strain ADP, AtzF, provides the final hydrolytic step for the mineralization of s-triazines, such as atrazine and cyanuric acid. Indeed, the action of AtzF provides metabolic access to two of the three nitrogens in each triazine ring. The X-ray structure of the N-terminal amidase domain of AtzF reveals that it is highly homologous to allophanate hydrolases involved in a different catabolic process in other organisms (i.e., the mineralization of urea). The smaller C-terminal domain does not appear to have a physiologically relevant catalytic function, as reported for the allophanate hydrolase of Kluyveromyces lactis, when purified enzyme was tested in vitro. However, the C-terminal domain does have a function in coordinating the quaternary structure of AtzF. Interestingly, we also show that AtzF forms a large, ca. 660-kDa, multienzyme complex with AtzD and AtzE that is capable of mineralizing cyanuric acid. The function of this complex may be to channel substrates from one active site to the next, effectively protecting unstable metabolites, such as allophanate, from solvent-mediated decarboxylation to a dead-end metabolic product.
Organizational Affiliation:
CSIRO Land and Water Flagship, Black Mountain, Canberra, ACT, Australia Research School of Chemistry, Australian National University, Canberra, ACT, Ausralia.