Design of Thermostable Rhamnogalacturonan Lyase Mutants from Bacillus Licheniformis by Combination of Targeted Single Point Mutations.
Silva, I.R., Jers, C., Otten, H., Nyffenegger, C., Larsen, D.M., Derkx, P.M.F., Meyer, A.S., Mikkelsen, J.D., Larsen, S.(2014) Appl Microbiol Biotechnol 98: 4521
- PubMed: 24419797
- DOI: https://doi.org/10.1007/s00253-013-5483-8
- Primary Citation of Related Structures:
4CAG - PubMed Abstract:
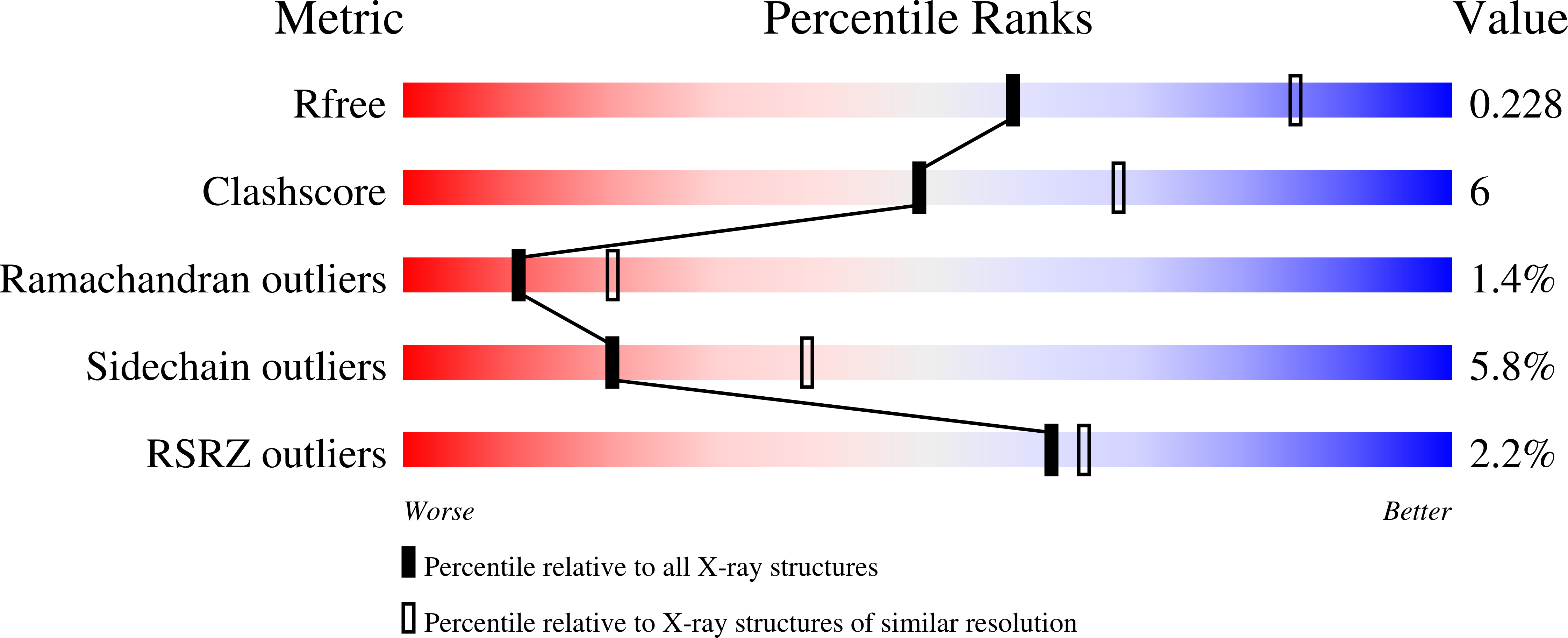
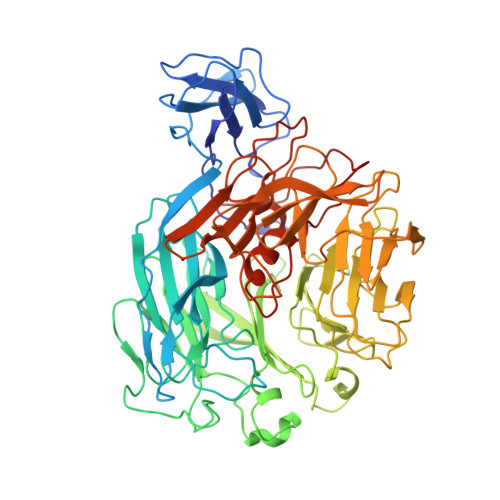
Rhamnogalacturonan I lyases (RGI lyases) (EC 4.2.2.-) catalyze cleavage of α-1,4 bonds between rhamnose and galacturonic acid in the backbone of pectins by β-elimination. In the present study, targeted improvement of the thermostability of a PL family 11 RGI lyase from Bacillus licheniformis (DSM 13/ATCC14580) was examined by using a combinatorial protein engineering approach exploring additive effects of single amino acid substitutions. These were selected by using a consensus approach together with assessing protein stability changes (PoPMuSiC) and B-factor iterative test (B-FIT). The second-generation mutants involved combinations of two to seven individually favorable single mutations. Thermal stability was examined as half-life at 60 °C and by recording of thermal transitions by circular dichroism. Surprisingly, the biggest increment in thermal stability was achieved by producing the wild-type RGI lyase in Bacillus subtilis as opposed to in Pichia pastoris; this effect is suggested to be a negative result of glycosylation of the P. pastoris expressed enzyme. A ~ twofold improvement in thermal stability at 60 °C, accompanied by less significant increases in T m of the enzyme mutants, were obtained due to additive stabilizing effects of single amino acid mutations (E434L, G55V, and G326E) compared to the wild type. The crystal structure of the B. licheniformis wild-type RGI lyase was also determined; the structural analysis corroborated that especially mutation of charged amino acids to hydrophobic ones in surface-exposed loops produced favorable thermal stability effects.
Organizational Affiliation:
Center for Bioprocess Engineering, Department of Chemical and Biochemical Engineering, Technical University of Denmark, Building 229, 2800, Kongens Lyngby, Denmark.