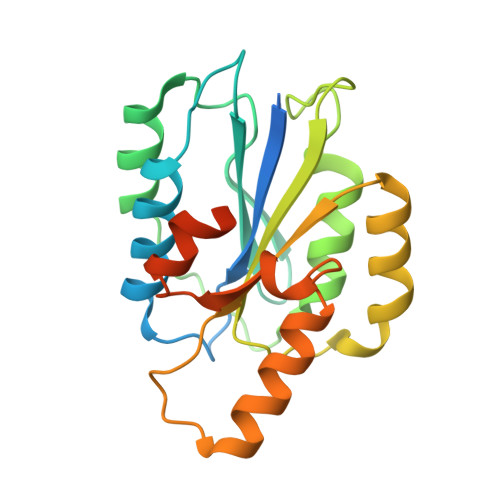
An Activating Mutation Reveals a Second Binding Mode of the Integrin Alpha2 I Domain to the Gfoger Motif in Collagens.
Carafoli, F., Hamaia, S.W., Bihan, D., Hohenester, E., Farndale, R.W.(2013) PLoS One 8: 69833
- PubMed: 23922814
- DOI: https://doi.org/10.1371/journal.pone.0069833
- Primary Citation of Related Structures:
4BJ3 - PubMed Abstract:
The GFOGER motif in collagens (O denotes hydroxyproline) represents a high-affinity binding site for all collagen-binding integrins. Other GxOGER motifs require integrin activation for maximal binding. The E318W mutant of the integrin α2β1 I domain displays a relaxed collagen specificity, typical of an active state. E318W binds more strongly than the wild-type α2 I domain to GMOGER, and forms a 2:1 complex with a homotrimeric, collagen-like, GFOGER peptide. Crystal structure analysis of this complex reveals two E318W I domains, A and B, bound to a single triple helix. The E318W I domains are virtually identical to the collagen-bound wild-type I domain, suggesting that the E318W mutation activates the I domain by destabilising the unligated conformation. E318W I domain A interacts with two collagen chains similarly to wild-type I domain (high-affinity mode). E318W I domain B makes favourable interactions with only one collagen chain (low-affinity mode). This observation suggests that single GxOGER motifs in the heterotrimeric collagens V and IX may support binding of activated integrins.
Organizational Affiliation:
Department of Life Sciences, Imperial College London, London, United Kingdom.