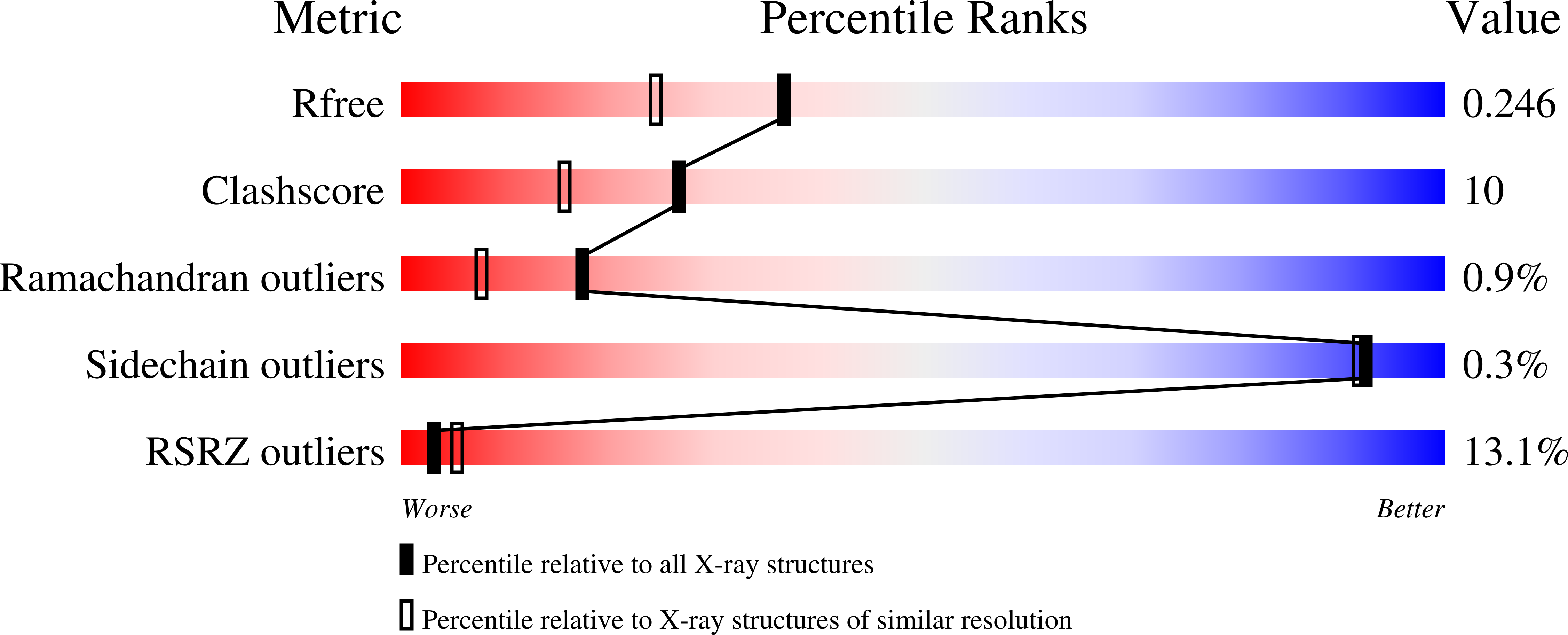
A Bimodular Mechanism of Calcium Control in Eukaryotes
Tidow, H., Poulsen, L.R., Andreeva, A., Knudsen, M., Hein, K.L., Wiuf, C., Palmgren, M.G., Nissen, P.(2012) Nature 491: 468
- PubMed: 23086147
- DOI: https://doi.org/10.1038/nature11539
- Primary Citation of Related Structures:
4AQR - PubMed Abstract:
Calcium ions (Ca(2+)) have an important role as secondary messengers in numerous signal transduction processes, and cells invest much energy in controlling and maintaining a steep gradient between intracellular (∼0.1-micromolar) and extracellular (∼2-millimolar) Ca(2+) concentrations. Calmodulin-stimulated calcium pumps, which include the plasma-membrane Ca(2+)-ATPases (PMCAs), are key regulators of intracellular Ca(2+) in eukaryotes. They contain a unique amino- or carboxy-terminal regulatory domain responsible for autoinhibition, and binding of calcium-loaded calmodulin to this domain releases autoinhibition and activates the pump. However, the structural basis for the activation mechanism is unknown and a key remaining question is how calmodulin-mediated PMCA regulation can cover both basal Ca(2+) levels in the nanomolar range as well as micromolar-range Ca(2+) transients generated by cell stimulation. Here we present an integrated study combining the determination of the high-resolution crystal structure of a PMCA regulatory-domain/calmodulin complex with in vivo characterization and biochemical, biophysical and bioinformatics data that provide mechanistic insights into a two-step PMCA activation mechanism mediated by calcium-loaded calmodulin. The structure shows the entire PMCA regulatory domain and reveals an unexpected 2:1 stoichiometry with two calcium-loaded calmodulin molecules binding to different sites on a long helix. A multifaceted characterization of the role of both sites leads to a general structural model for calmodulin-mediated regulation of PMCAs that allows stringent, highly responsive control of intracellular calcium in eukaryotes, making it possible to maintain a stable, basal level at a threshold Ca(2+) concentration, where steep activation occurs.
Organizational Affiliation:
Centre for Membrane Pumps in Cells and Disease - PUMPKIN, Aarhus University, Gustav Wieds Vej 10c, DK-8000 Aarhus C, Denmark.