Structure-Guided Mutations in the Terminal Organelle Protein MG491 Cause Major Motility and Morphologic Alterations on Mycoplasma genitalium.
Martinelli, L., Garcia-Morales, L., Querol, E., Pinol, J., Fita, I., Calisto, B.M.(2016) PLoS Pathog 12: e1005533-e1005533
- PubMed: 27082435
- DOI: https://doi.org/10.1371/journal.ppat.1005533
- Primary Citation of Related Structures:
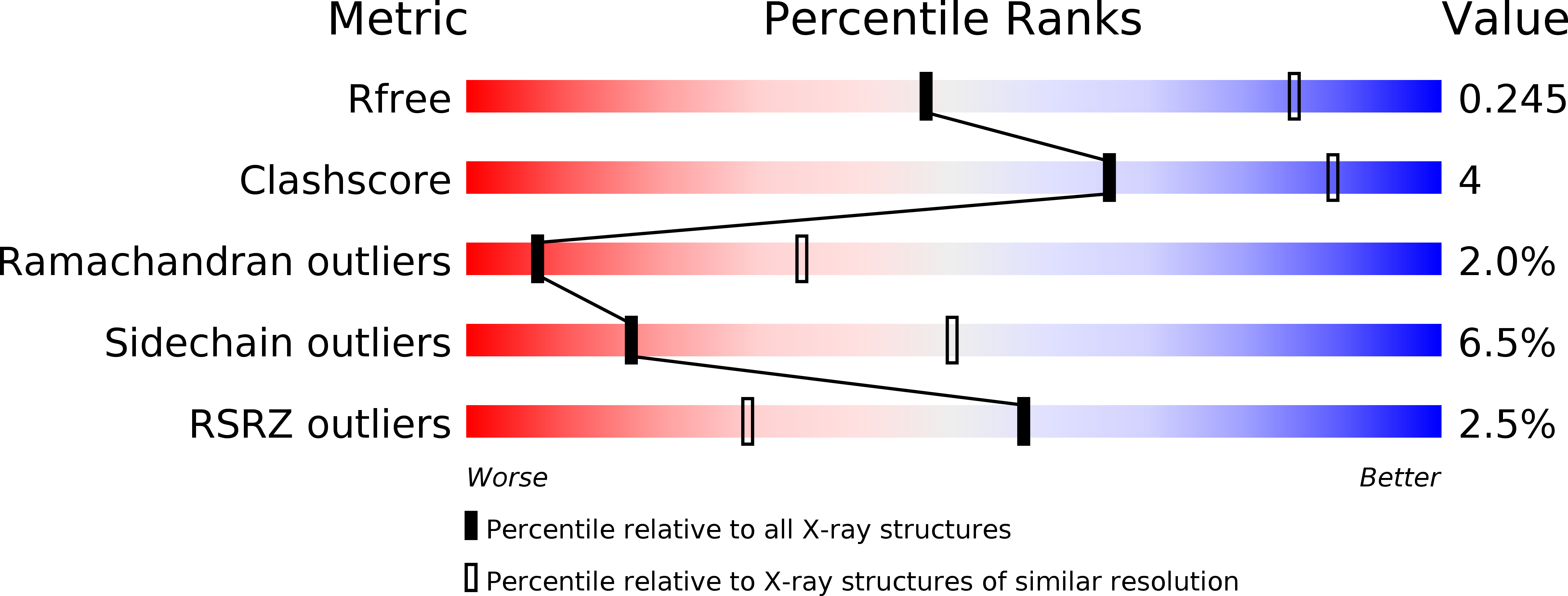
4XNG - PubMed Abstract:
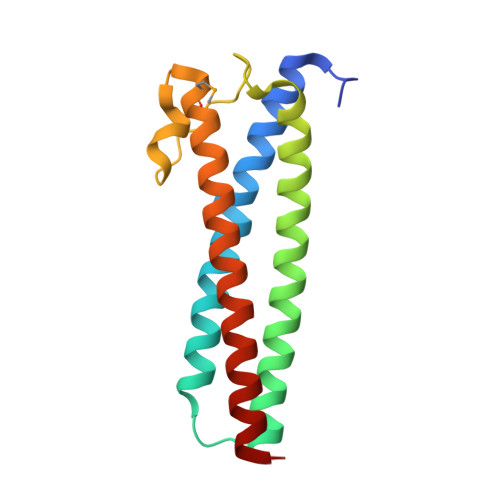
The emergent human pathogen Mycoplasma genitalium, with one of the smallest genomes among cells capable of growing in axenic cultures, presents a flask-shaped morphology due to a protrusion of the cell membrane, known as the terminal organelle, that is involved in cell adhesion and motility and is an important virulence factor of this microorganism. The terminal organelle is supported by a cytoskeleton complex of about 300 nm in length that includes three substructures: the terminal button, the rod and the wheel complex. The crystal structure of the MG491 protein, a proposed component of the wheel complex, has been determined at ~3 Å resolution. MG491 subunits are composed of a 60-residue N-terminus, a central three-helix-bundle spanning about 150 residues and a C-terminal region that appears to be quite flexible and contains the region that interacts with MG200, another key protein of the terminal organelle. The MG491 molecule is a tetramer presenting a unique organization as a dimer of asymmetric pairs of subunits. The asymmetric arrangement results in two very different intersubunit interfaces between the central three-helix-bundle domains, which correlates with the formation of only ~50% of the intersubunit disulfide bridges of the single cysteine residue found in MG491 (Cys87). Moreover, M. genitalium cells with a point mutation in the MG491 gene causing the change of Cys87 to Ser present a drastic reduction in motility (as determined by microcinematography) and important alterations in morphology (as determined by electron microscopy), while preserving normal levels of the terminal organelle proteins. Other variants of MG491, designed also according to the structural information, altered significantly the motility and/or the cell morphology. Together, these results indicate that MG491 plays a key role in the functioning, organization and stabilization of the terminal organelle.
Organizational Affiliation:
Instituto de Biología Molecular de Barcelona (IBMB-CSIC), Parc Científic de Barcelona, Barcelona, Spain.