Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase.
Rui, Z., Li, X., Zhu, X., Liu, J., Domigan, B., Barr, I., Cate, J.H., Zhang, W.(2014) Proc Natl Acad Sci U S A 111: 18237-18242
- PubMed: 25489112
- DOI: https://doi.org/10.1073/pnas.1419701112
- Primary Citation of Related Structures:
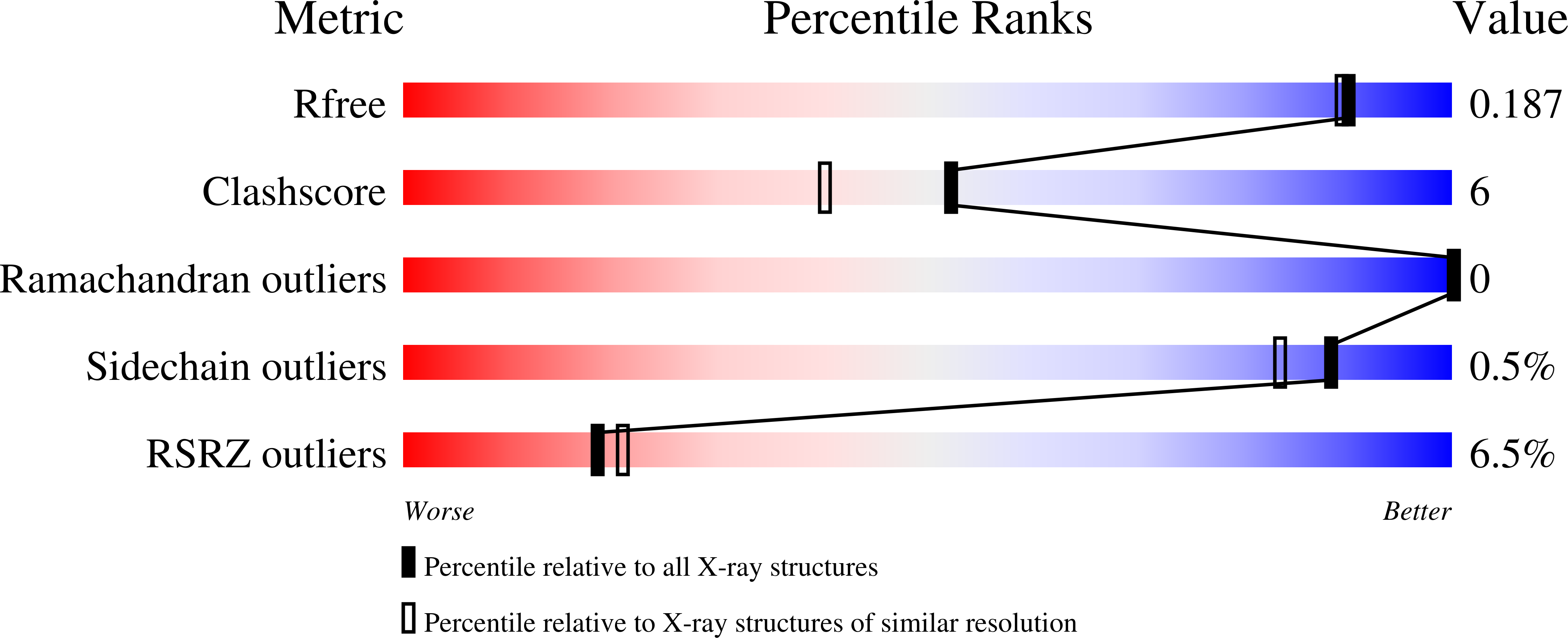
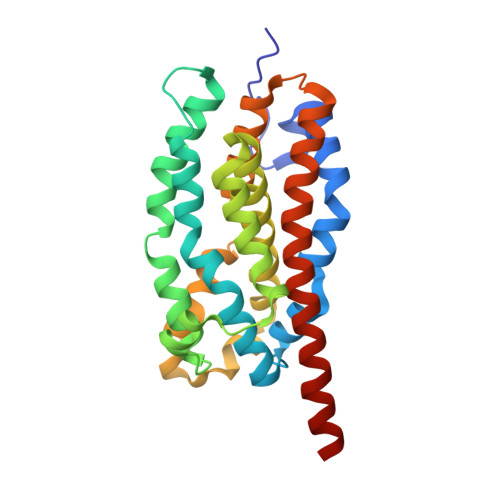
4WWJ, 4WWZ, 4WX0 - PubMed Abstract:
Aliphatic medium-chain 1-alkenes (MCAEs, ∼10 carbons) are "drop-in" compatible next-generation fuels and precursors to commodity chemicals. Mass production of MCAEs from renewable resources holds promise for mitigating dependence on fossil hydrocarbons. An MCAE, such as 1-undecene, is naturally produced by Pseudomonas as a semivolatile metabolite through an unknown biosynthetic pathway. We describe here the discovery of a single gene conserved in Pseudomonas responsible for 1-undecene biosynthesis. The encoded enzyme is able to convert medium-chain fatty acids (C10-C14) into their corresponding terminal olefins using an oxygen-activating, nonheme iron-dependent mechanism. Both biochemical and X-ray crystal structural analyses suggest an unusual mechanism of β-hydrogen abstraction during fatty acid substrate activation. Our discovery unveils previously unidentified chemistry in the nonheme Fe(II) enzyme family, provides an opportunity to explore the biology of 1-undecene in Pseudomonas, and paves the way for tailored bioconversion of renewable raw materials to MCAE-based biofuels and chemical commodities.
Organizational Affiliation:
Energy Biosciences Institute, Departments of Chemical and Biomolecular Engineering.