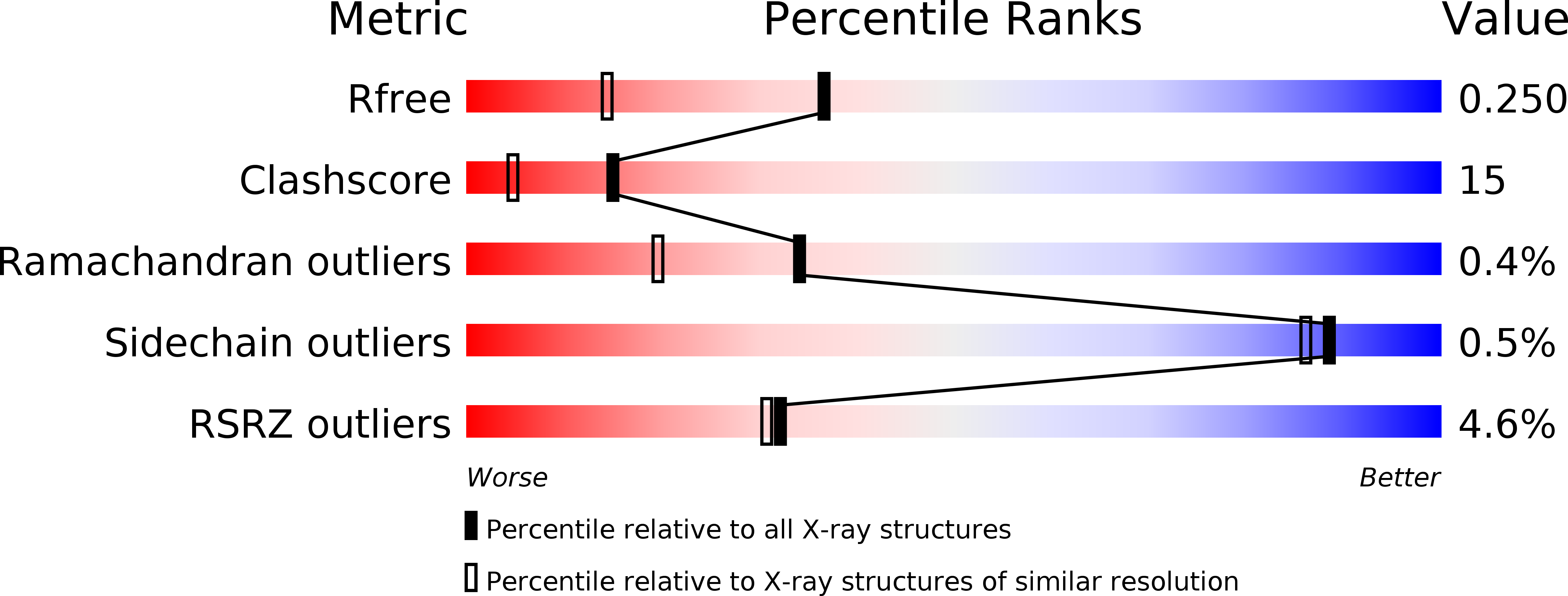
Crystal structure of ATP-binding subunit of an ABC transporter from Geobacillus kaustophilus.
Manjula, M., Pampa, K.J., Kumar, S.M., Mukherjee, S., Kunishima, N., Rangappa, K.S., Lokanath, N.K.(2015) Biochem Biophys Res Commun 459: 113-117
- PubMed: 25724946
- DOI: https://doi.org/10.1016/j.bbrc.2015.02.079
- Primary Citation of Related Structures:
4RVC - PubMed Abstract:
The ATP binding cassette (ABC) transporters, represent one of the largest superfamilies of primary transporters, which are very essential for various biological functions. The crystal structure of ATP-binding subunit of an ABC transporter from Geobacillus kaustophilus has been determined at 1.77 Å resolution. The crystal structure revealed that the protomer has two thick arms, (arm I and II), which resemble 'L' shape. The ATP-binding pocket is located close to the end of arm I. ATP molecule is docked into the active site of the protein. The dimeric crystal structure of ATP-binding subunit of ABC transporter from G. kaustophilus has been compared with the previously reported crystal structure of ATP-binding subunit of ABC transporter from Salmonella typhimurium.
Organizational Affiliation:
Department of Studies in Physics, University of Mysore, Mysore 570 006, India.