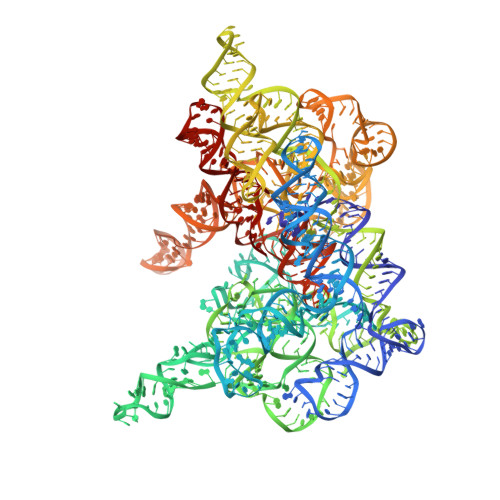
Crystal structure of a eukaryotic group II intron lariat.
Robart, A.R., Chan, R.T., Peters, J.K., Rajashankar, K.R., Toor, N.(2014) Nature 514: 193-197
- PubMed: 25252982
- DOI: https://doi.org/10.1038/nature13790
- Primary Citation of Related Structures:
4R0D - PubMed Abstract:
The formation of branched lariat RNA is an evolutionarily conserved feature of splicing reactions for both group II and spliceosomal introns. The lariat is important for the fidelity of 5' splice-site selection and consists of a 2'-5' phosphodiester bond between a bulged adenosine and the 5' end of the intron. To gain insight into this ubiquitous intramolecular linkage, we determined the crystal structure of a eukaryotic group IIB intron in the lariat form at 3.7 Å. This revealed that two tandem tetraloop-receptor interactions, η-η' and π-π', place domain VI in the core to position the lariat bond in the post-catalytic state. On the basis of structural and biochemical data, we propose that π-π' is a dynamic interaction that mediates the transition between the two steps of splicing, with η-η' serving an ancillary role. The structure also reveals a four-magnesium-ion cluster involved in both catalysis and positioning of the 5' end. Given the evolutionary relationship between group II and nuclear introns, it is likely that this active site configuration exists in the spliceosome as well.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, California 92093, USA.