Structural bases of norovirus RNA dependent RNA polymerase inhibition by novel suramin-related compounds.
Croci, R., Pezzullo, M., Tarantino, D., Milani, M., Tsay, S.C., Sureshbabu, R., Tsai, Y.J., Mastrangelo, E., Rohayem, J., Bolognesi, M., Hwu, J.R.(2014) PLoS One 9: e91765-e91765
- PubMed: 24622391
- DOI: https://doi.org/10.1371/journal.pone.0091765
- Primary Citation of Related Structures:
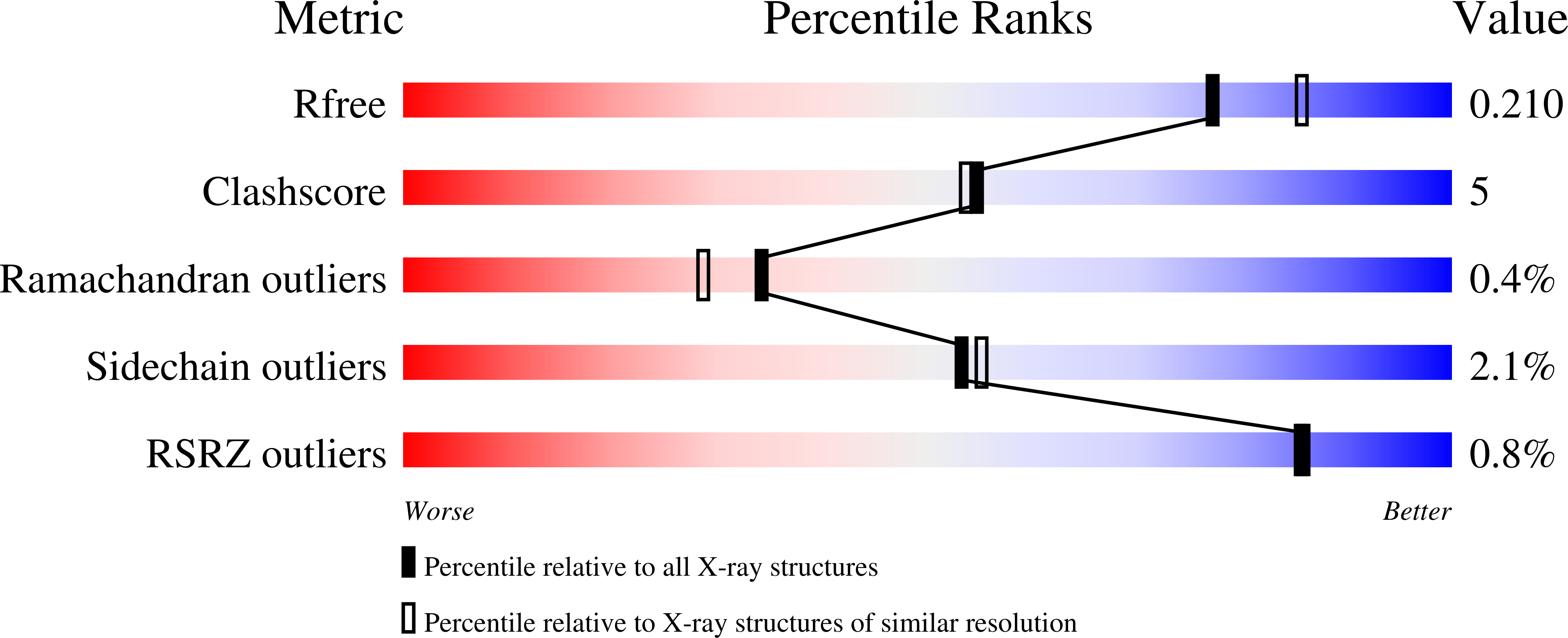
4NRT, 4NRU - PubMed Abstract:
Noroviruses (NV) are +ssRNA viruses responsible for severe gastroenteritis; no effective vaccines/antivirals are currently available. We previously identified Suramin (9) as a potent inhibitor of NV-RNA dependent RNA polymerase (NV-RdRp). Despite significant in vitro activities versus several pharmacological targets, Suramin clinical use is hampered by pharmacokinetics/toxicity problems. To improve Suramin access to NV-RdRp in vivo, a Suramin-derivative, 8, devoid of two sulphonate groups, was synthesized, achieving significant anti-human-NV-RdRp activity (IC50 = 28 nM); the compound inhibits also murine NV (mNV) RdRp. The synthesis process led to the isolation/characterization of lower molecular weight intermediates (3-7) hosting only one sulphonate head. The crystal structures of both hNV/mNV-RdRps in complex with 6, were analyzed, providing new knowledge on the interactions that a small fragment can establish with NV-RdRps, and establishing a platform for structure-guided optimization of potency, selectivity and drugability.
Organizational Affiliation:
Department of BioSciences, University of Milano, Milano, Italy.