Sister Chromatid Cohesion Establishment Factor ESCO1 Operates by Substrate-Assisted Catalysis.
Kouznetsova, E., Kanno, T., Karlberg, T., Thorsell, A.G., Wisniewska, M., Kursula, P., Sjogren, C., Schuler, H.(2016) Structure 24: 789-796
- PubMed: 27112597
- DOI: https://doi.org/10.1016/j.str.2016.03.021
- Primary Citation of Related Structures:
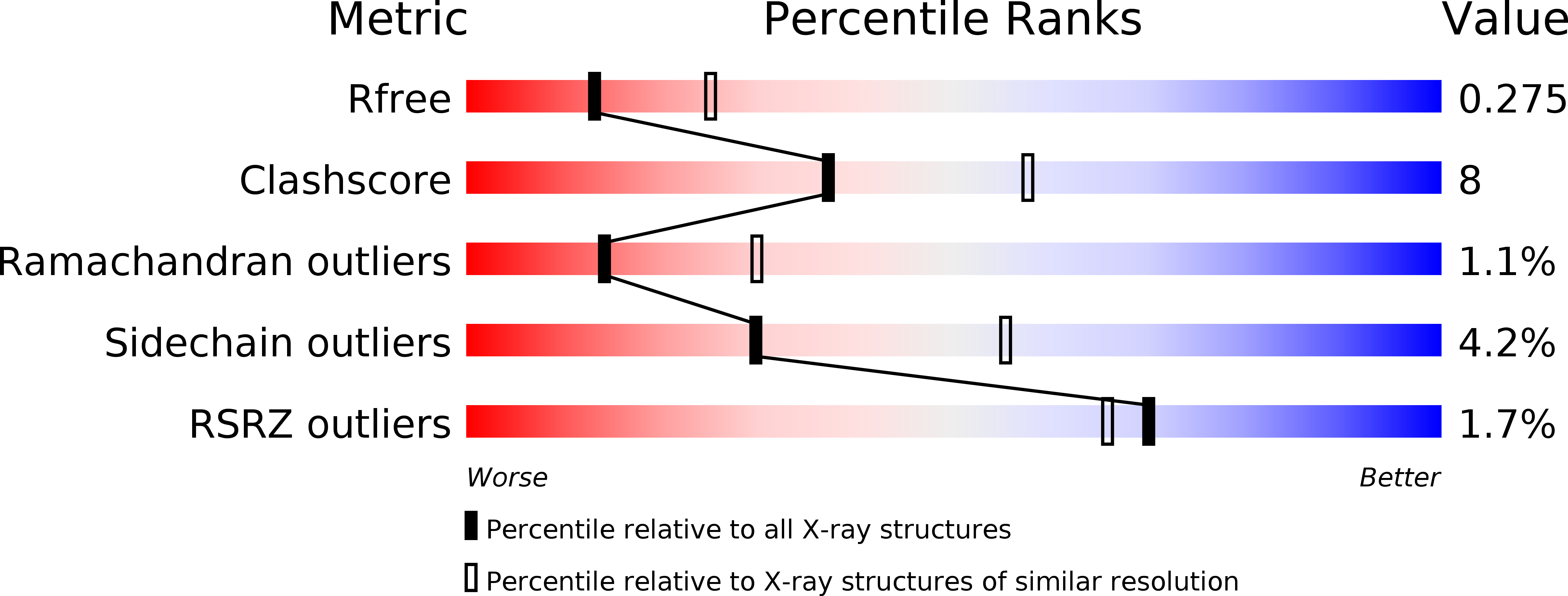
4MXE - PubMed Abstract:
Sister chromatid cohesion, formed by the cohesin protein complex, is essential for chromosome segregation. In order for cohesion to be established, the cohesin subunit SMC3 needs to be acetylated by a homolog of the ESCO1/Eco1 acetyltransferases, the enzymatic mechanism of which has remained unknown. Here we report the crystal structure of the ESCO1 acetyltransferase domain in complex with acetyl-coenzyme A, and show by SAXS that ESCO1 is a dimer in solution. The structure reveals an active site that lacks a potential catalytic base side chain. However, mutation of glutamate 789, a surface residue that is close to the automodification target lysine 803, strongly reduces autoacetylation of ESCO1. Moreover, budding yeast Smc3 mutated at the conserved residue D114, adjacent to the cohesion-activating acetylation site K112,K113, cannot be acetylated in vivo. This indicates that ESCO1 controls cohesion through substrate-assisted catalysis. Thus, this study discloses a key mechanism for cohesion establishment.
Organizational Affiliation:
Structural Genomics Consortium and Department of Medical Biochemistry and Biophysics, Karolinska Institutet, 17177 Stockholm, Sweden.