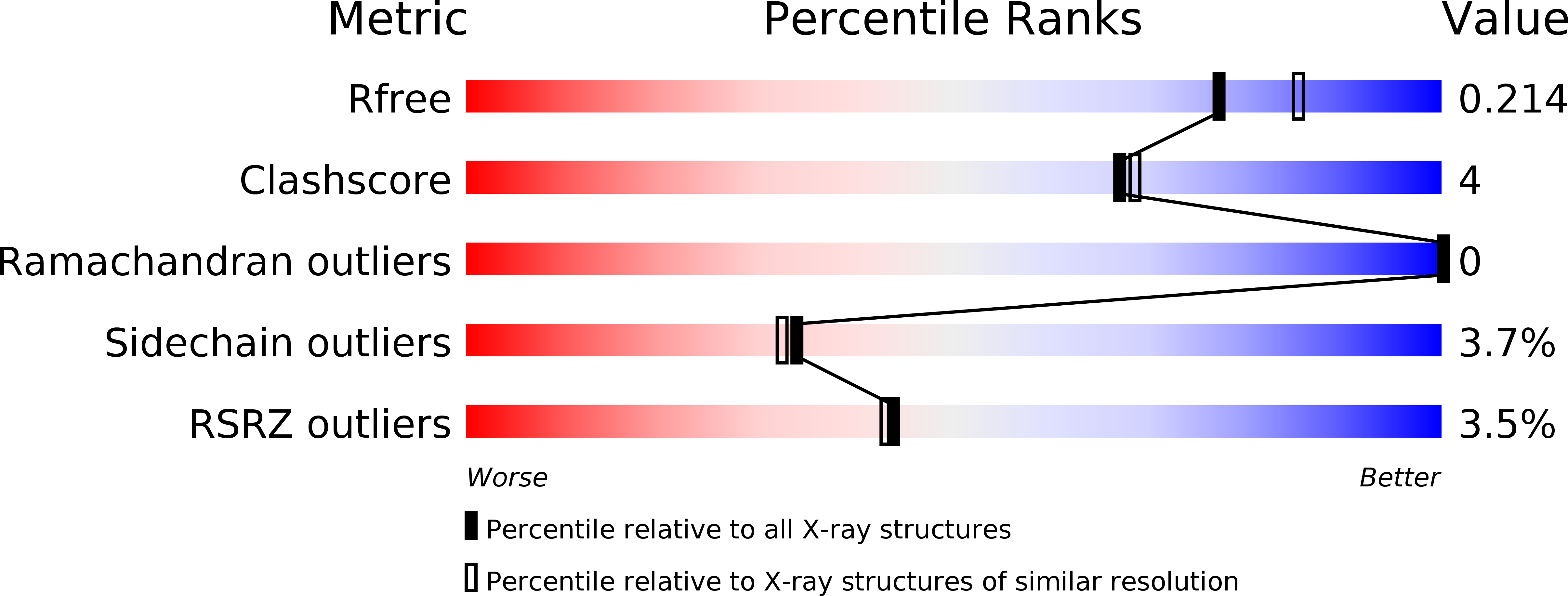
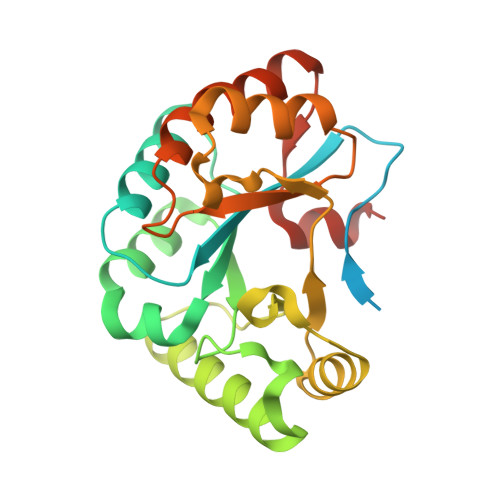
Structure determination of BA0150, a putative polysaccharide deacetylase from Bacillus anthracis.
Strunk, R.J., Piemonte, K.M., Petersen, N.M., Koutsioulis, D., Bouriotis, V., Perry, K., Cole, K.E.(2014) Acta Crystallogr Sect F Struct Biol Cryst Commun 70: 156-159
- PubMed: 24637747
- DOI: https://doi.org/10.1107/S2053230X13034262
- Primary Citation of Related Structures:
4M1B - PubMed Abstract:
Polysaccharide deacetylases are bacterial enzymes that catalyze the deacetylation of acetylated sugars on the membranes of Gram-positive bacteria, allowing them to be unrecognized by host immune systems. Inhibition of these enzymes would disrupt such pathogenic defensive mechanisms and therefore offers a promising route for the development of novel antibiotic therapeutics. Here, the first X-ray crystal structure of BA0150, a putative polysaccharide deacetylase from Bacillus anthracis, is reported to 2.0 Å resolution. The overall structure maintains the conserved (α/β)8 fold that is characteristic of this family of enzymes. The lack of a catalytic metal ion and a distinctive metal-binding site, however, suggest that this enzyme is not a functional polysaccharide deacetylase.
Organizational Affiliation:
Department of Chemistry, Ithaca College, 953 Danby Road, Ithaca, NY 14850, USA.