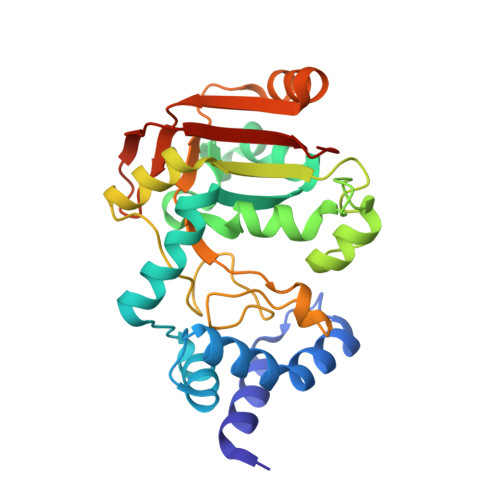
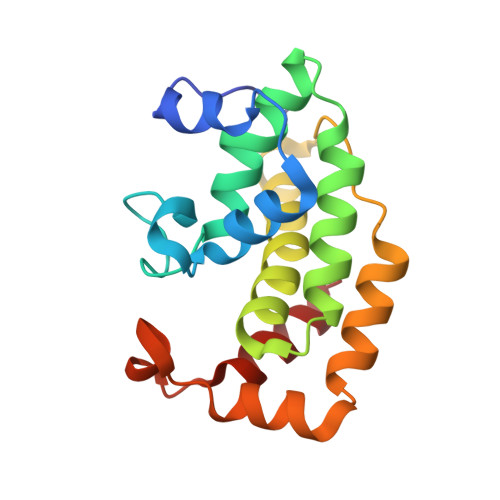
High-resolution crystal structure of Streptococcus pyogenes beta-NAD(+) glycohydrolase in complex with its endogenous inhibitor IFS reveals a highly water-rich interface
Yoon, J.Y., An, D.R., Yoon, H.-J., Kim, H.S., Lee, S.J., Im, H.N., Jang, J.Y., Suh, S.W.(2013) J Synchrotron Radiat 20: 962-967
- PubMed: 24121349
- DOI: https://doi.org/10.1107/S0909049513020803
- Primary Citation of Related Structures:
4KT6 - PubMed Abstract:
One of the virulence factors produced by Streptococcus pyogenes is β-NAD(+) glycohydrolase (SPN). S. pyogenes injects SPN into the cytosol of an infected host cell using the cytolysin-mediated translocation pathway. As SPN is toxic to bacterial cells themselves, S. pyogenes possesses the ifs gene that encodes an endogenous inhibitor for SPN (IFS). IFS is localized intracellularly and forms a complex with SPN. This intracellular complex must be dissociated during export through the cell envelope. To provide a structural basis for understanding the interactions between SPN and IFS, the complex was overexpressed between the mature SPN (residues 38-451) and the full-length IFS (residues 1-161), but it could not be crystallized. Therefore, limited proteolysis was used to isolate a crystallizable SPNct-IFS complex, which consists of the SPN C-terminal domain (SPNct; residues 193-451) and the full-length IFS. Its crystal structure has been determined by single anomalous diffraction and the model refined at 1.70 Å resolution. Interestingly, our high-resolution structure of the complex reveals that the interface between SPNct and IFS is highly rich in water molecules and many of the interactions are water-mediated. The wet interface may facilitate the dissociation of the complex for translocation across the cell envelope.
Organizational Affiliation:
Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Republic of Korea.