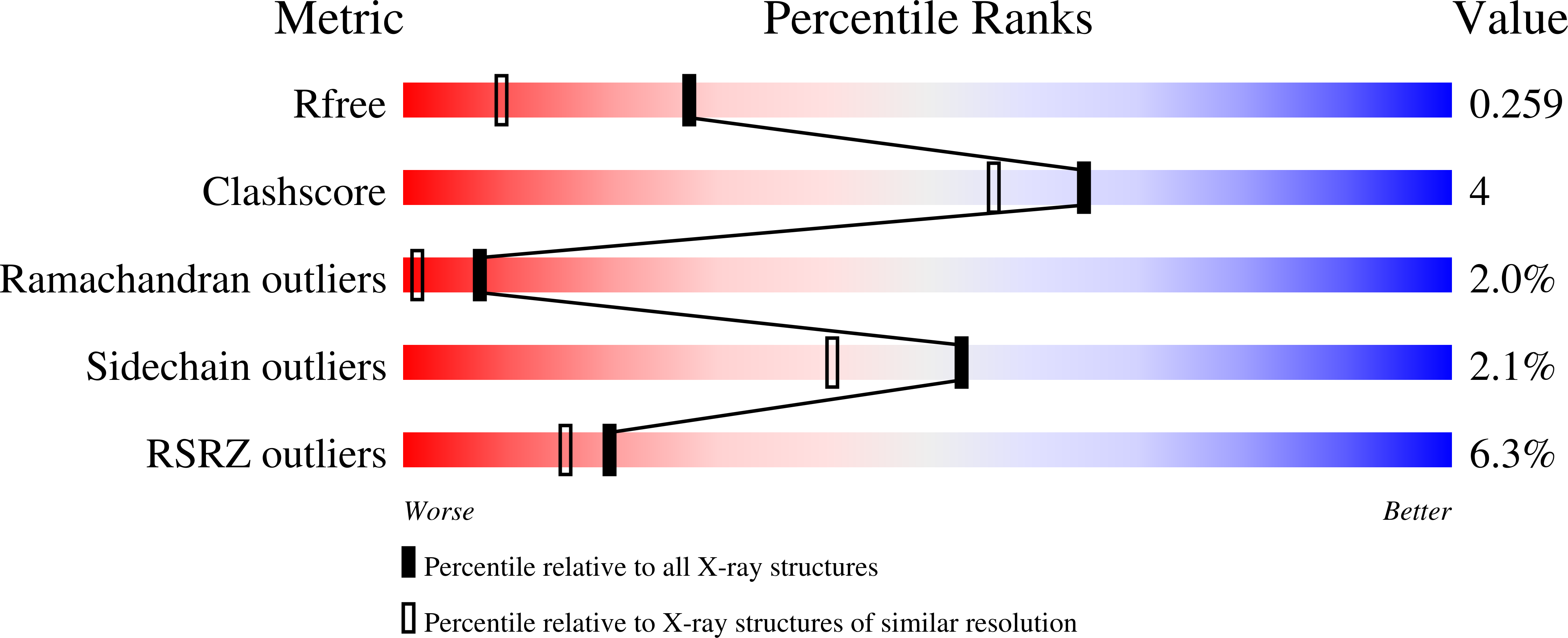
Mapping Hfq-RNA interaction surfaces using tryptophan fluorescence quenching.
Robinson, K.E., Orans, J., Kovach, A.R., Link, T.M., Brennan, R.G.(2014) Nucleic Acids Res 42: 2736-2749
- PubMed: 24288369
- DOI: https://doi.org/10.1093/nar/gkt1171
- Primary Citation of Related Structures:
4JLI, 4JRI, 4JRK, 4JUV - PubMed Abstract:
Hfq is a posttranscriptional riboregulator and RNA chaperone that binds small RNAs and target mRNAs to effect their annealing and message-specific regulation in response to environmental stressors. Structures of Hfq-RNA complexes indicate that U-rich sequences prefer the proximal face and A-rich sequences the distal face; however, the Hfq-binding sites of most RNAs are unknown. Here, we present an Hfq-RNA mapping approach that uses single tryptophan-substituted Hfq proteins, all of which retain the wild-type Hfq structure, and tryptophan fluorescence quenching (TFQ) by proximal RNA binding. TFQ properly identified the respective distal and proximal binding of A15 and U6 RNA to Gram-negative Escherichia coli (Ec) Hfq and the distal face binding of (AA)3A, (AU)3A and (AC)3A to Gram-positive Staphylococcus aureus (Sa) Hfq. The inability of (GU)3G to bind the distal face of Sa Hfq reveals the (R-L)n binding motif is a more restrictive (A-L)n binding motif. Remarkably Hfq from Gram-positive Listeria monocytogenes (Lm) binds (GU)3G on its proximal face. TFQ experiments also revealed the Ec Hfq (A-R-N)n distal face-binding motif should be redefined as an (A-A-N)n binding motif. TFQ data also demonstrated that the 5'-untranslated region of hfq mRNA binds both the proximal and distal faces of Ec Hfq and the unstructured C-terminus.
Organizational Affiliation:
Department of Biochemistry, Duke University, Durham, NC 27710, USA and Department of Biochemistry and Molecular Biology, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.