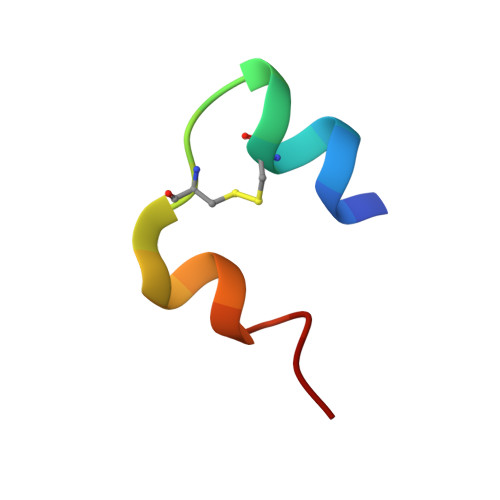
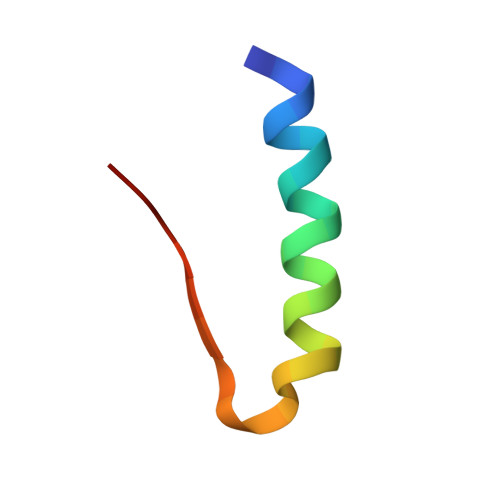
A T3R3 hexamer of the human insulin variant B28Asp.
Palmieri, L.C., Favero-Retto, M.P., Lourenco, D., Lima, L.M.(2013) Biophys Chem 173: 1-7
- PubMed: 23428413
- DOI: https://doi.org/10.1016/j.bpc.2013.01.003
- Primary Citation of Related Structures:
4GBC, 4GBI, 4GBK, 4GBL, 4GBN - PubMed Abstract:
Insulin shows a complex equilibrium between monomers and hexamers, involving varying conformers and association states. We sought to perform a structural characterization of the fast-acting human insulin variant B28Asp ("aspart"). Small-angle X-ray scattering measurements reveal similar globular behavior in both the aspart and regular human insulin, with a Rg of 19Å and a Dmax of approximately 50Å, indicating similar mean quaternary assembly distribution. Crystallographic assays revealed a T3R3 assembly of the aspart insulin formed by the TR dimer in the asymmetric unit, with all the first 8 residues of the B chain in the R-state monomer in helical conformation and the participation of its B3Asn in the stabilization of the hexamer. Our data provide access to novel structural information on aspart insulin such as an aspart insulin dimer in solution, the aspart insulin in T conformation and a pure R-state conformer establishing a T3R3 assembly, providing further insight on the stepwise conformational transition and assembly of this fast-insulin.
Organizational Affiliation:
School of Pharmacy, Federal University of Rio de Janeiro - UFRJ. CCS, Bss34, Ilha do Fundão, 21941-590, Rio de Janeiro, RJ, Brazil.