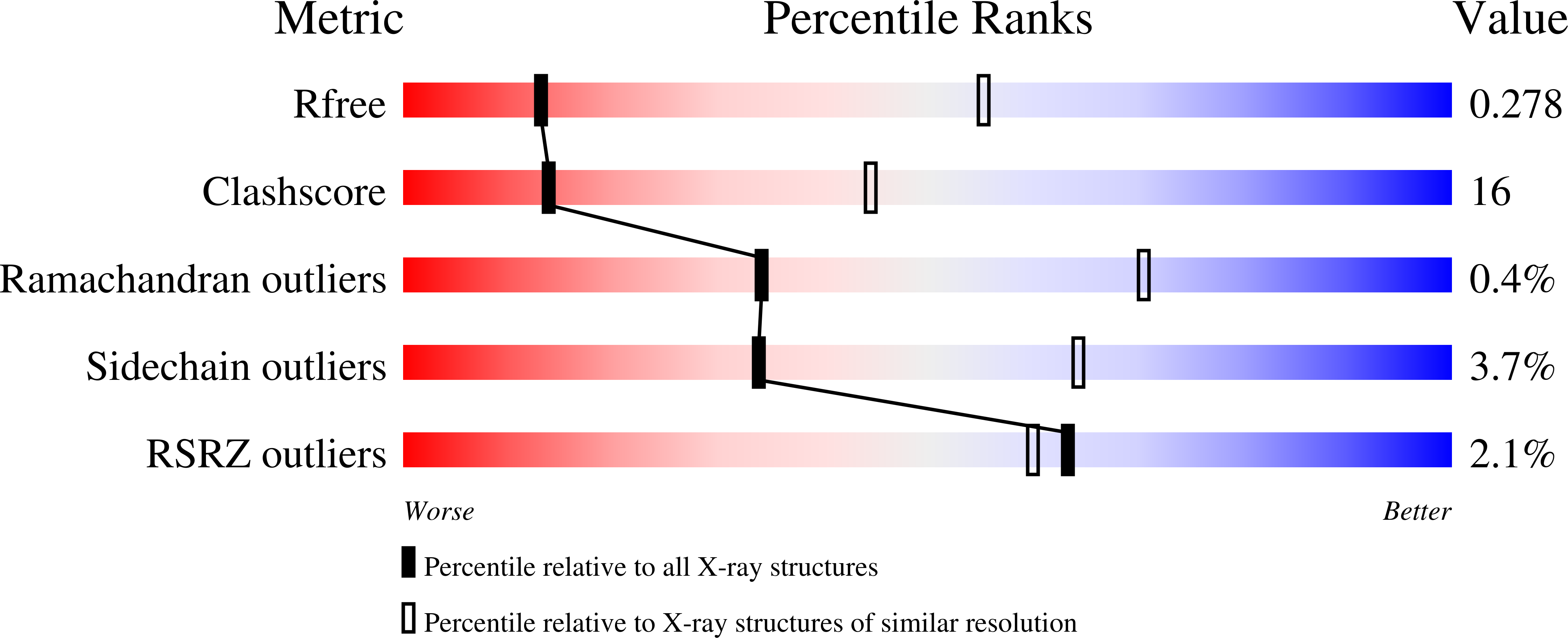
Structural Insights Into Oligomerization and Mitochondrial Remodelling of Dynamin 1-Like Protein.
Frohlich, C., Grabiger, S., Schwefel, D., Faelber, K., Rosenbaum, E., Mears, J., Rocks, O., Daumke, O.(2013) EMBO J 32: 1280
- PubMed: 23584531
- DOI: https://doi.org/10.1038/emboj.2013.74
- Primary Citation of Related Structures:
4BEJ - PubMed Abstract:
Dynamin 1-like protein (DNM1L) mediates fission of mitochondria and peroxisomes, and dysfunction of DNM1L has been implicated in several neurological disorders. To study the molecular basis of mitochondrial remodelling, we determined the crystal structure of DNM1L that is comprised of a G domain, a bundle signalling element and a stalk. DNM1L assembled via a central stalk interface, and mutations in this interface disrupted dimerization and interfered with membrane binding and mitochondrial targeting. Two sequence stretches at the tip of the stalk were shown to be required for ordered assembly of DNM1L on membranes and its function in mitochondrial fission. In the crystals, DNM1L dimers further assembled via a second, previously undescribed, stalk interface to form a linear filament. Mutations in this interface interfered with liposome tubulation and mitochondrial remodelling. Based on these results and electron microscopy reconstructions, we propose an oligomerization mode for DNM1L which differs from that of dynamin and might be adapted to the remodelling of mitochondria.
Organizational Affiliation:
Crystallography, Max-Delbrück-Center for Molecular Medicine, Berlin, Germany.