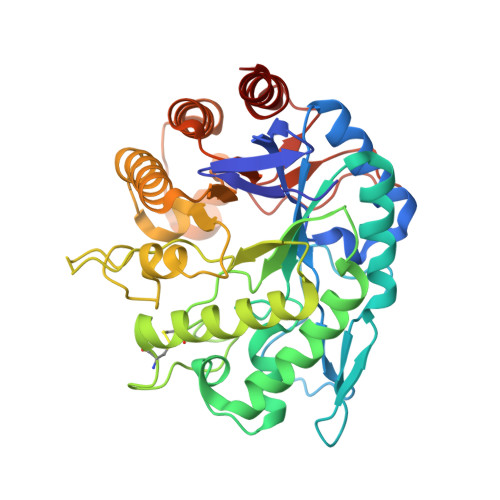
Endo-Beta-D-1,4-Mannanase from Chrysonilia Sitophila Displays a Novel Loop Arrangement for Substrate Selectivity
Goncalves, A.M.D., Silva, C.S., Madeira, T.I., Coelho, R., De Sanctis, D., San Romao, M.V., Bento, I.(2012) Acta Crystallogr D Biol Crystallogr 68: 1468
- PubMed: 23090396
- DOI: https://doi.org/10.1107/S0907444912034646
- Primary Citation of Related Structures:
4AWE - PubMed Abstract:
The crystal structure of wild-type endo-β-D-1,4-mannanase (EC 3.2.1.78) from the ascomycete Chrysonilia sitophila (CsMan5) has been solved at 1.40 Å resolution. The enzyme isolated directly from the source shows mixed activity as both an endo-glucanase and an endo-mannanase. CsMan5 adopts the (β/α)(8)-barrel fold that is well conserved within the GH5 family and has highest sequence and structural homology to the GH5 endo-mannanases. Superimposition with proteins of this family shows a unique structural arrangement of three surface loops of CsMan5 that stretch over the active centre, promoting an altered topography of the binding cleft. The most relevant feature results from the repositioning of a long loop at the extremity of the binding cleft, resulting in a shortened glycone-binding region with two subsites. The other two extended loops flanking the binding groove produce a narrower cleft compared with the wide architecture observed in GH5 homologues. Two aglycone subsites (+1 and +2) are identified and a nonconserved tryptophan (Trp271) at the +1 subsite may offer steric hindrance. Taken together, these findings suggest that the discrimination of mannan substrates is achieved through modified loop length and structure.
Organizational Affiliation:
Macromolecular Crystallography Unit, Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, Avenida da República, 2780-157 Oeiras, Portugal.