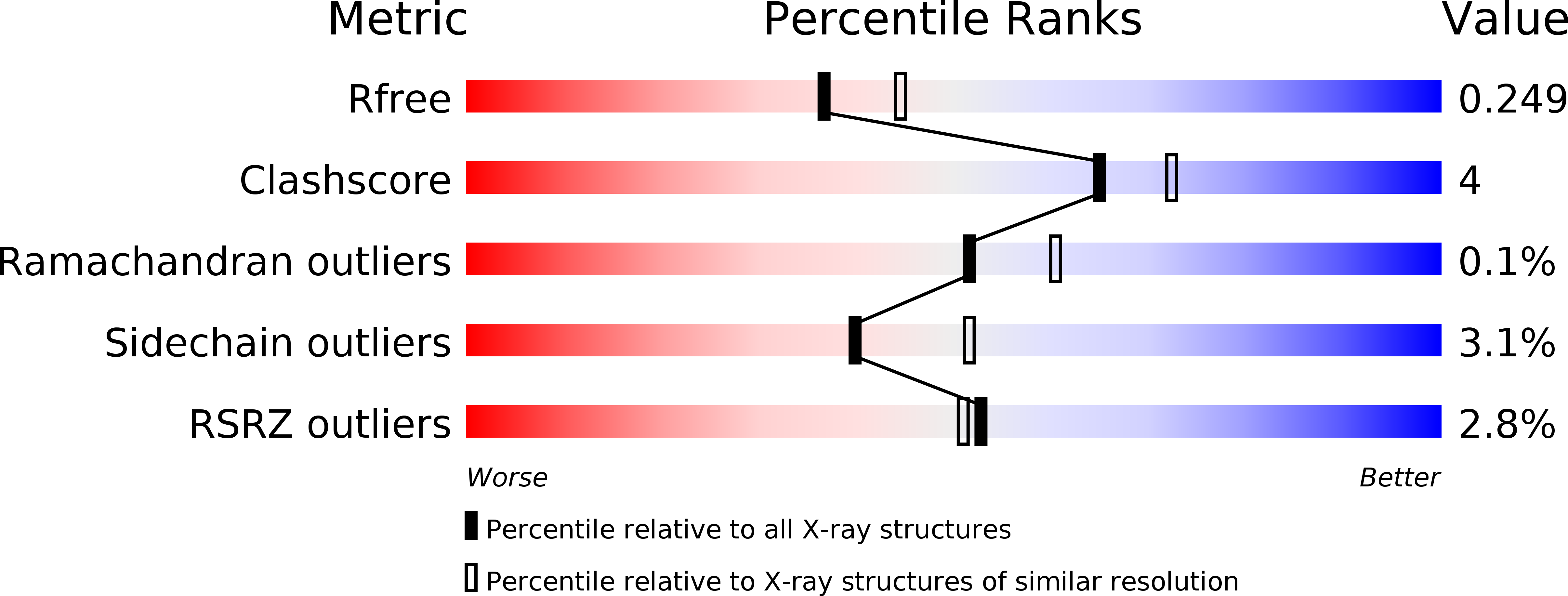
Structure of Diaminohydroxyphosphoribosylaminopyrimidine Deaminase/5-Amino-6-(5-Phosphoribosylamino)Uracil Reductase from Acinetobacter Baumannii.
Dawson, A., Trumper, P., Chrysostomou, G., Hunter, W.N.(2013) Acta Crystallogr Sect F Struct Biol Cryst Commun 69: 611
- PubMed: 23722836
- DOI: https://doi.org/10.1107/S174430911301292X
- Primary Citation of Related Structures:
3ZPC, 3ZPG - PubMed Abstract:
The bifunctional diaminohydroxyphosphoribosylaminopyrimidine deaminase/5-amino-6-(5-phosphoribosylamino)uracil reductase (RibD) represents a potential antibacterial drug target. The structure of recombinant Acinetobacter baumannii RibD is reported in orthorhombic and tetragonal crystal forms at 2.2 and 2.0 Å resolution, respectively. Comparisons with orthologous structures in the Protein Data Bank indicated close similarities. The tetragonal crystal form was obtained in the presence of guanosine monophosphate, which surprisingly was observed to occupy the adenine-binding site of the reductase domain.
Organizational Affiliation:
Division of Biological Chemistry and Drug Discovery, College of Life Sciences, University of Dundee, Dundee DD1 5EH, Scotland.