Cryo-Em Structures of Two Bovine Adenovirus Type 3 Intermediates
Cheng, L., Huang, X., Li, X., Xiong, W., Sun, W., Yang, C., Zhang, K., Wang, Y., Liu, H., Huang, X., Ji, G., Sun, F., Zheng, C., Zhu, P.(2014) Virology 450: 174
- PubMed: 24503080
- DOI: https://doi.org/10.1016/j.virol.2013.12.012
- Primary Citation of Related Structures:
3ZIF - PubMed Abstract:
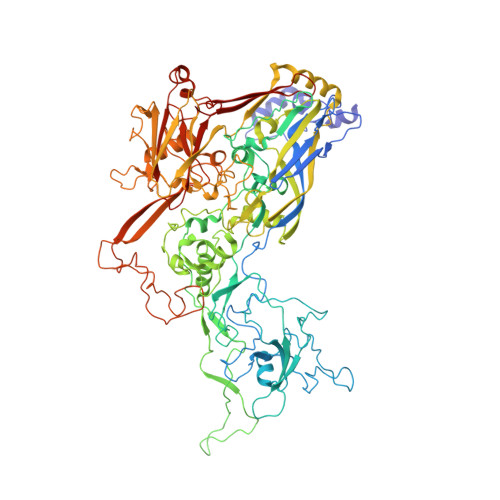
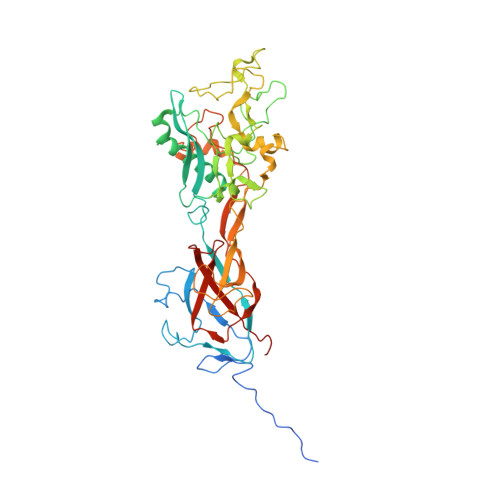
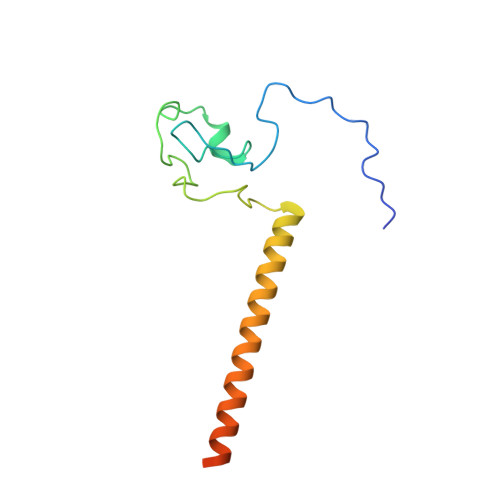
Adenoviruses (Ads) infect hosts from all vertebrate species and have been investigated as vaccine vectors. We report here near-atomic structures of two bovine Ad type 3 (BAd3) intermediates obtained by cryo-electron microscopy. A comparison between the two intermediate structures reveals that the differences are localized in the fivefold vertex region, while their facet structures are identical. The overall facet structure of BAd3 exhibits a similar structure to human Ads; however, BAd3 protein IX has a unique conformation. Mass spectrometry and cryo-electron tomography analyses indicate that one intermediate structure represents the stage during DNA encapsidation, whilst the other intermediate structure represents a later stage. These results also suggest that cleavage of precursor protein VI occurs during, rather than after, the DNA encapsidation process. Overall, our results provide insights into the mechanism of Ad assembly, and allow the first structural comparison between human and nonhuman Ads at backbone level.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.