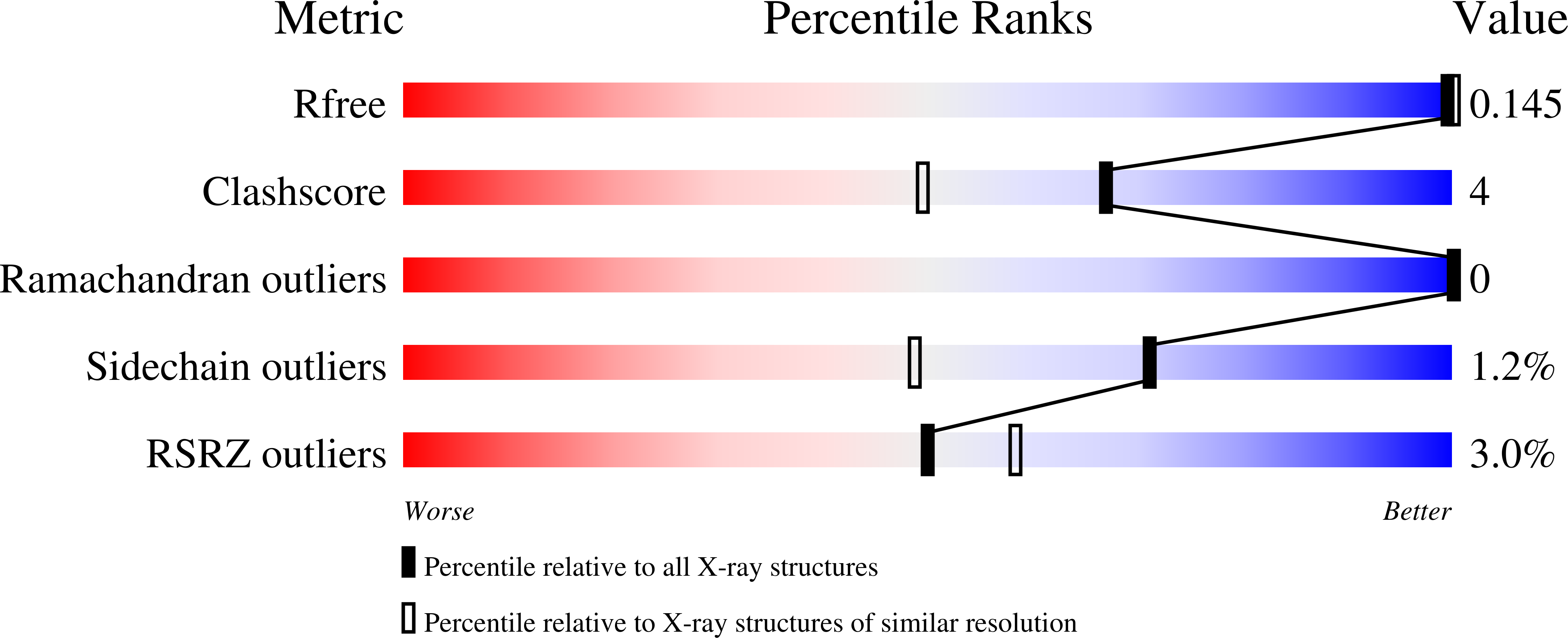
High-temperature and high-resolution crystallography of thermostable copper nitrite reductase.
Fukuda, Y., Inoue, T.(2015) Chem Commun (Camb) 51: 6532-6535
- PubMed: 25563214
- DOI: https://doi.org/10.1039/c4cc09553g
- Primary Citation of Related Structures:
3X1N - PubMed Abstract:
The 1.55 Å resolution thermostable copper nitrite reductase structure at 320 K displayed a near-bidentate binding mode of nitrite distinct from a monodentate mode in a cryogenic structure. To our knowledge, this is the first case in which the difference in substrate binding modes between cryogenic and high-temperature structures has been visualized by crystallography.
Organizational Affiliation:
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. y_fukuda@chem.eng.osaka-u.ac.jp.