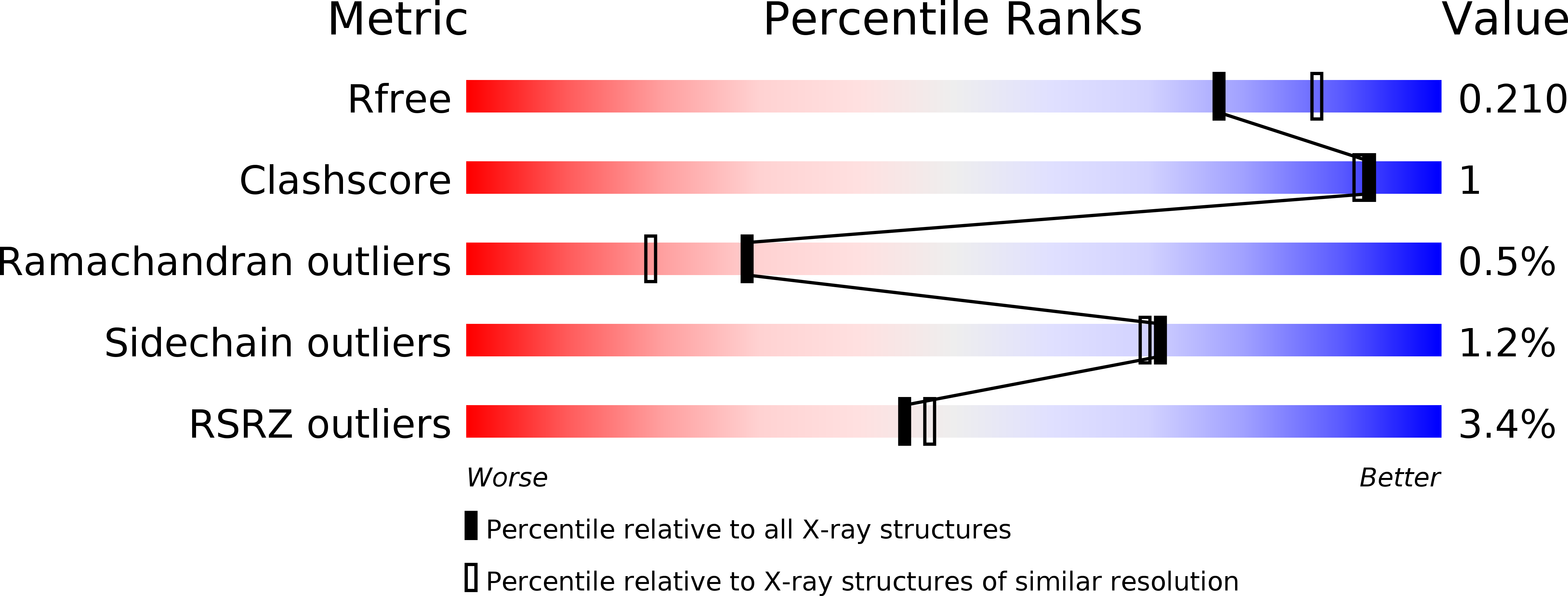
Structure of monomeric Na-GST-3, a glutathione S-transferase from the major human hookworm parasite Necator americanus.
Kelleher, A., Zhan, B., Asojo, O.A.(2013) Acta Crystallogr Sect F Struct Biol Cryst Commun 69: 839-843
- PubMed: 23908024
- DOI: https://doi.org/10.1107/S1744309113017661
- Primary Citation of Related Structures:
3W8S - PubMed Abstract:
Necator americanus is the major cause of human hookworm infection, which is a global cause of anemia in the developing world. Ongoing efforts to control hookworm infection include the identification of candidate vaccine antigens as well as potential therapeutic targets from the infective L3 larval stages and adult stages of the parasite. One promising family of proteins are the adult-stage-secreted cytosolic glutathione S-transferases (GSTs). Nematode GSTs facilitate the inactivation and degradation of a variety of electrophilic substrates (drugs) via the nucleophilic addition of reduced glutathione. Parasite GSTs also play significant roles in multi-drug resistance and the modulation of host immune defense mechanisms. Here, the structure of Na-GST-3, one of three GSTs secreted by adult-stage N. americanus, is reported. Unlike most GST structures, the Na-GST-3 crystal contains a monomer in the asymmetric unit. However, the monomer forms a prototypical GST dimer across the crystallographic twofold. A glutathione from the fermentation process is bound to the monomer. The overall binding cavity of Na-GST-3 is reminiscent of that of other N. americanus GSTs and is larger and capable of binding a wider array of ligands than GSTs from organisms that have other major detoxifying mechanisms. Furthermore, despite having low sequence identity to the host GST, Na-GST-3 has a greater tertiary-structure similarity to human sigma-class GST than was observed for the other N. americanus GSTs.
Organizational Affiliation:
Department of Pediatrics, Baylor College of Medicine, 1102 Bates Avenue BCM 320, Houston, TX 77030, USA.