Identification of the SSB-interaction platform of Deinococcus radiodurans uracil-DNA glycosylase
George, N.P., Keck, J.L.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uracil-DNA glycosylase | 255 | Deinococcus radiodurans R1 = ATCC 13939 = DSM 20539 | Mutation(s): 0 Gene Names: DR0689, DR_0689, ung EC: 3.2.2.27 | 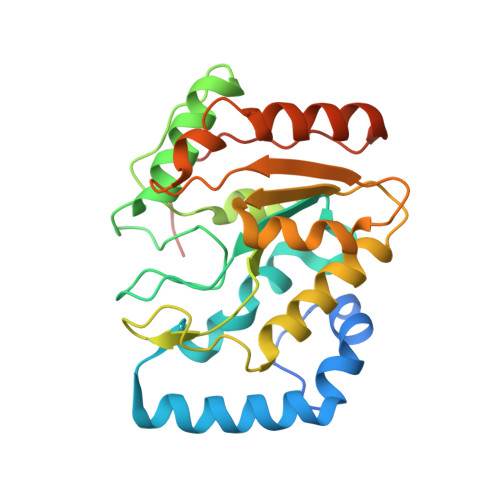 | |
UniProt | |||||
Find proteins for Q9RWH9 (Deinococcus radiodurans (strain ATCC 13939 / DSM 20539 / JCM 16871 / CCUG 27074 / LMG 4051 / NBRC 15346 / NCIMB 9279 / VKM B-1422 / R1)) Explore Q9RWH9 Go to UniProtKB: Q9RWH9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9RWH9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Single-stranded DNA-binding protein | 12 | Deinococcus radiodurans | Mutation(s): 0 |  | |
UniProt | |||||
Find proteins for Q9RY51 (Deinococcus radiodurans (strain ATCC 13939 / DSM 20539 / JCM 16871 / CCUG 27074 / LMG 4051 / NBRC 15346 / NCIMB 9279 / VKM B-1422 / R1)) Explore Q9RY51 Go to UniProtKB: Q9RY51 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9RY51 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| IOD Query on IOD | C [auth A], D [auth A] | IODIDE ION I XMBWDFGMSWQBCA-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 78.343 | α = 90 |
| b = 92.64 | β = 90 |
| c = 66.012 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data collection |