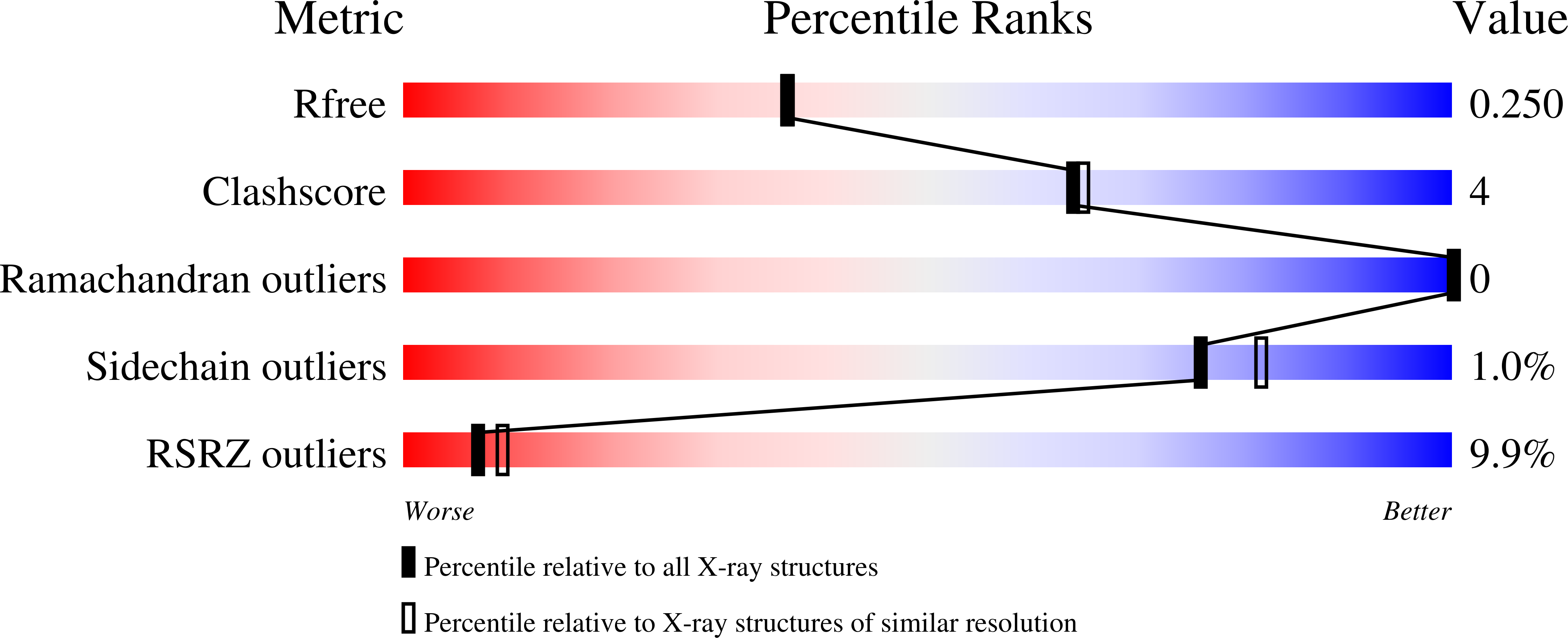
Structural and functional characterization of Helicobacter pylori DsbG
Yoon, J.Y., Kim, J., Lee, S.J., Kim, H.S., Im, H.N., Yoon, H., Kim, K.H., Kim, S., Han, B.W., Suh, S.W.(2011) FEBS Lett 585: 3862-3867
- PubMed: 22062156
- DOI: https://doi.org/10.1016/j.febslet.2011.10.042
- Primary Citation of Related Structures:
3TDG - PubMed Abstract:
Dsb proteins play important roles in bacterial pathogenicity. To better understand the role of Dsb proteins in Helicobacter pylori, we have structurally and functionally characterized H. pylori DsbG (HP0231). The monomer consists of two domains connected by a helical linker. Two monomers associate to form a V-shaped dimer. The monomeric and dimeric structures of H. pylori DsbG show significant differences compared to Escherichia coli DsbG. Two polyethylene glycol molecules are bound in the cleft of the V-shaped dimer, suggesting a possible role as a chaperone. Furthermore, we show that H. pylori DsbG functions as a reductase against HP0518, a putative L,D-transpeptidase with a catalytic cysteine residue.
Organizational Affiliation:
Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul, Republic of Korea.