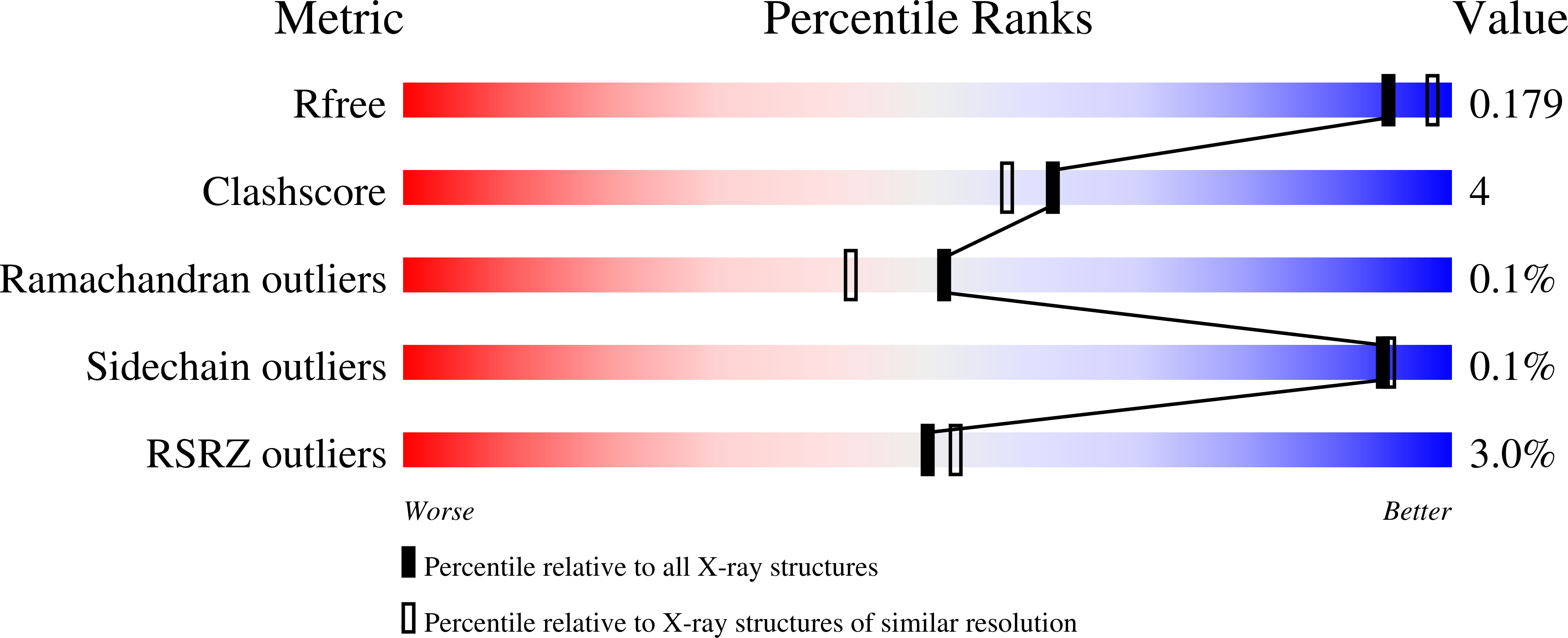
A novel inhibitor of Plasmodium falciparum spermidine synthase: a twist in the tail.
Burger, P.B., Williams, M., Sprenger, J., Reeksting, S.B., Botha, M., Muller, I.B., Joubert, F., Birkholtz, L.M., Louw, A.I.(2015) Malar J 14: 54-54
- PubMed: 25651815
- DOI: https://doi.org/10.1186/s12936-015-0572-z
- Primary Citation of Related Structures:
3RIE - PubMed Abstract:
Plasmodium falciparum is the most pathogenic of the human malaria parasite species and a major cause of death in Africa. It's resistance to most of the current drugs accentuates the pressing need for new chemotherapies. Polyamine metabolism of the parasite is distinct from the human pathway making it an attractive target for chemotherapeutic development. Plasmodium falciparum spermidine synthase (PfSpdS) catalyzes the synthesis of spermidine and spermine. It is a major polyamine flux-determining enzyme and spermidine is a prerequisite for the post-translational activation of P. falciparum eukaryotic translation initiation factor 5A (elF5A). The most potent inhibitors of eukaryotic SpdS's are not specific for PfSpdS.
Organizational Affiliation:
Department of Biochemistry, UP Centre for Sustainable Malaria Control, Faculty of Natural and Agricultural Sciences, University of Pretoria, Private Bag X20, Hatfield, 0028, South Africa. pieter.burger@gmail.com.