Structural biochemistry of nuclear actin-related proteins 4 and 8 reveals their interaction with actin.
Fenn, S., Breitsprecher, D., Gerhold, C.B., Witte, G., Faix, J., Hopfner, K.P.(2011) EMBO J 30: 2153-2166
- PubMed: 21499228
- DOI: https://doi.org/10.1038/emboj.2011.118
- Primary Citation of Related Structures:
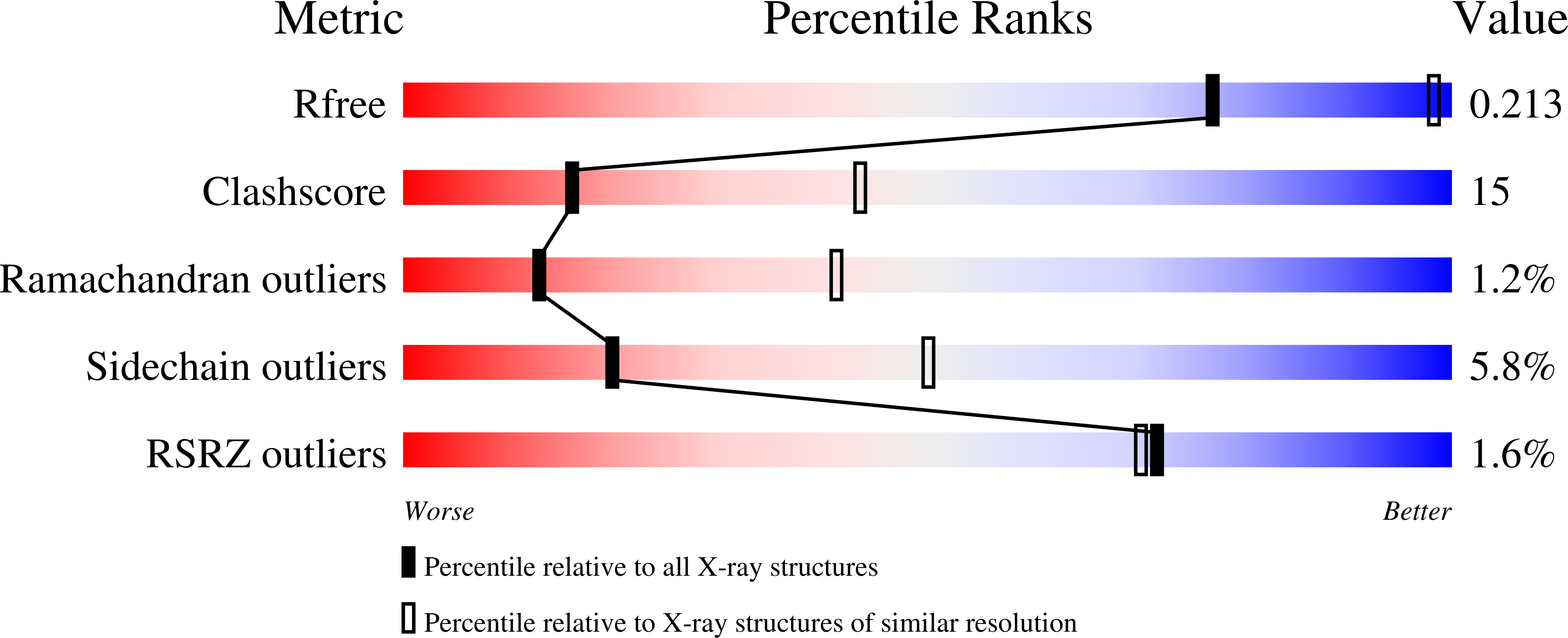
3QB0 - PubMed Abstract:
Nuclear actin and actin-related proteins (Arps) are integral components of various chromatin-remodelling complexes. Actin in such nuclear assemblies does not form filaments but associates in defined complexes, for instance with Arp4 and Arp8 in the INO80 remodeller. To understand the relationship between nuclear actin and its associated Arps and to test the possibility that Arp4 and Arp8 help maintain actin in defined states, we structurally analysed Arp4 and Arp8 from Saccharomyces cerevisiae and tested their biochemical effects on actin assembly and disassembly. The solution structures of isolated Arp4 and Arp8 indicate them to be monomeric and the crystal structure of ATP-Arp4 reveals several differences to actin that explain why Arp4 does not form filaments itself. Remarkably, Arp4, assisted by Arp8, influences actin polymerization in vitro and is able to depolymerize actin filaments. Arp4 likely forms a complex with monomeric actin via the barbed end. Our data thus help explaining how nuclear actin is held in a discrete complex within the INO80 chromatin remodeller.
Organizational Affiliation:
Department of Biochemistry, Gene Center of the Ludwig-Maximilians-University Munich, Munich, Germany.