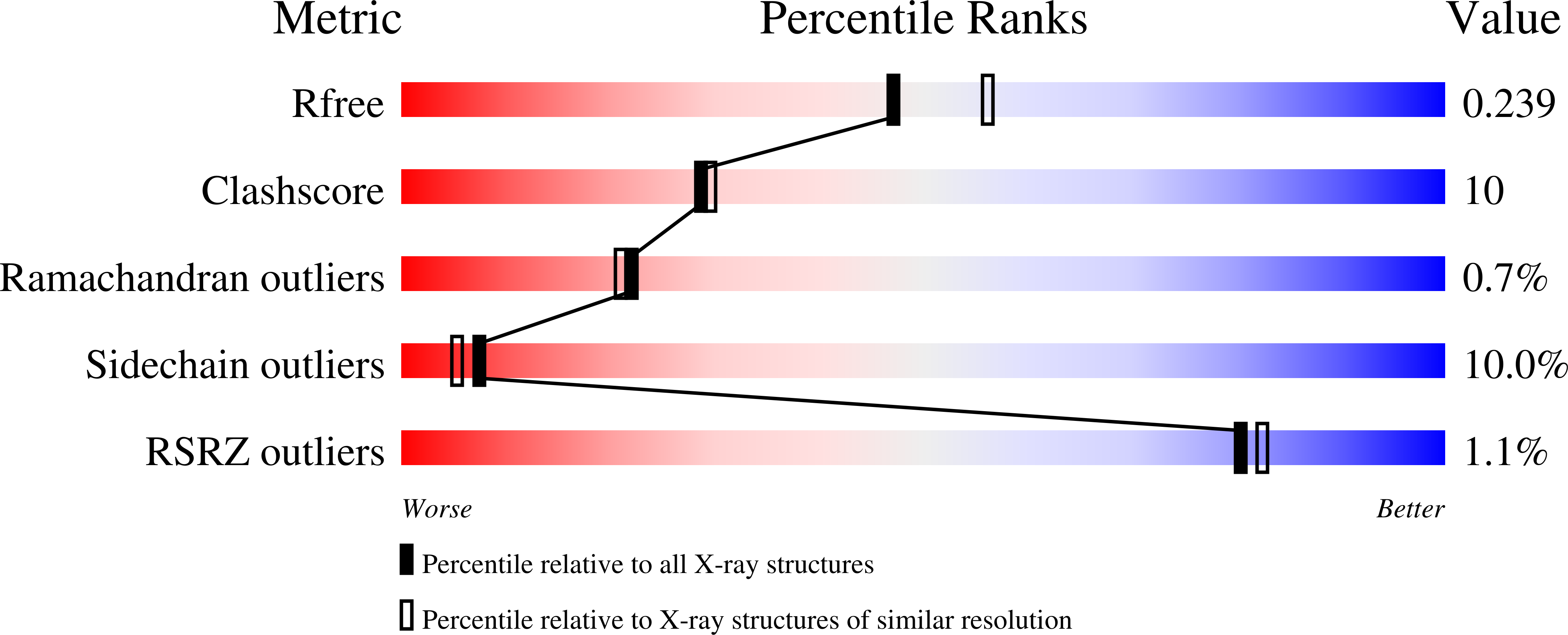
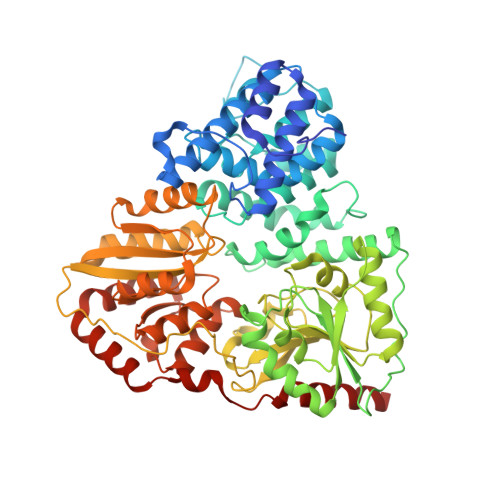
Structural Insights into the Glycosyltransferase Activity of the Actinobacillus pleuropneumoniae HMW1C-like Protein.
Kawai, F., Grass, S., Kim, Y., Choi, K.J., St Geme, J.W., Yeo, H.J.(2011) J Biol Chem 286: 38546-38557
- PubMed: 21908603
- DOI: https://doi.org/10.1074/jbc.M111.237602
- Primary Citation of Related Structures:
3Q3E, 3Q3H, 3Q3I - PubMed Abstract:
Glycosylation of proteins is a fundamental process that influences protein function. The Haemophilus influenzae HMW1 adhesin is an N-linked glycoprotein that mediates adherence to respiratory epithelium, an essential early step in the pathogenesis of H. influenzae disease. HMW1 is glycosylated by HMW1C, a novel glycosyltransferase in the GT41 family that creates N-glycosidic linkages with glucose and galactose at asparagine residues and di-glucose linkages at sites of glucose modification. Here we report the crystal structure of Actinobacillus pleuropneumoniae HMW1C (ApHMW1C), a functional homolog of HMW1C. The structure of ApHMW1C contains an N-terminal all α-domain (AAD) fold and a C-terminal GT-B fold with two Rossmann-like domains and lacks the tetratricopeptide repeat fold characteristic of the GT41 family. The GT-B fold harbors the binding site for UDP-hexose, and the interface of the AAD fold and the GT-B fold forms a unique groove with potential to accommodate the acceptor protein. Structure-based functional analyses demonstrated that the HMW1C protein shares the same structure as ApHMW1C and provided insights into the unique bi-functional activity of HMW1C and ApHMW1C, suggesting an explanation for the similarities and differences of the HMW1C-like proteins compared with other GT41 family members.
Organizational Affiliation:
Department of Biology and Biochemistry, University of Houston, Houston, Texas 77204.