A Model for Group B Streptococcus Pilus Type 1: The Structure of a 35-kDa C-Terminal Fragment of the Major Pilin GBS80.
Vengadesan, K., Ma, X., Dwivedi, P., Ton-That, H., Narayana, S.V.L.(2011) J Mol Biol 407: 731-743
- PubMed: 21333654
- DOI: https://doi.org/10.1016/j.jmb.2011.02.024
- Primary Citation of Related Structures:
3PF2, 3PG2 - PubMed Abstract:
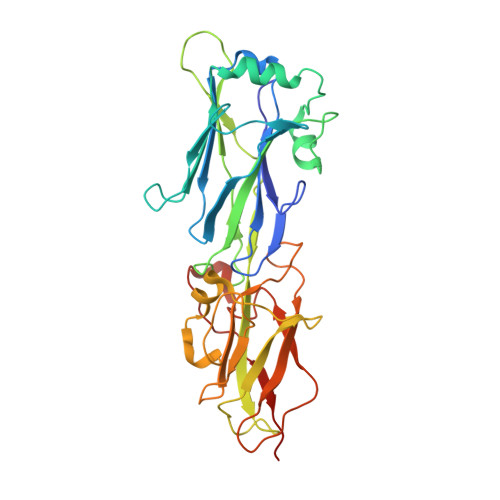
The Gram-positive pathogen Streptococcus agalactiae, known as group B Streptococcus (GBS), is the leading cause of bacterial septicemia, pneumonia, and meningitis among neonates. GBS assembles two types of pili-pilus islands (PIs) 1 and 2-on its surface to adhere to host cells and to initiate colonization for pathogenesis. The GBS PI-1 pilus is made of one major pilin, GBS80, which forms the pilus shaft, and two secondary pilins, GBS104 and GBS52, which are incorporated into the pilus at various places. We report here the crystal structure of the 35-kDa C-terminal fragment from GBS80, which is composed of two IgG-like domains (N2-N3). The structure was solved by single-wavelength anomalous dispersion using sodium-iodide-soaked crystals and diffraction data collected at the home source. The N2 domain exhibits a cnaA/DEv-IgG fold with two calcium-binding sites, while the N3 domain displays a cnaB/IgG-rev fold. We have built a model for full-length GBS80 (N1, N2, and N3) with the help of available homologous major pilin structures, and we propose a model for the GBS PI-1 pilus shaft. The N2 and N3 domains are arranged in tandem along the pilus shaft, whereas the respective N1 domain is tilted by approximately 20° away from the pilus axis. We have also identified a pilin-like motif in the minor pilin GBS52, which might aid its incorporation at the pilus base.
Organizational Affiliation:
Center for Biophysical Sciences and Engineering, School of Optometry, University of Alabama at Birmingham, Birmingham, AL 35294, USA.