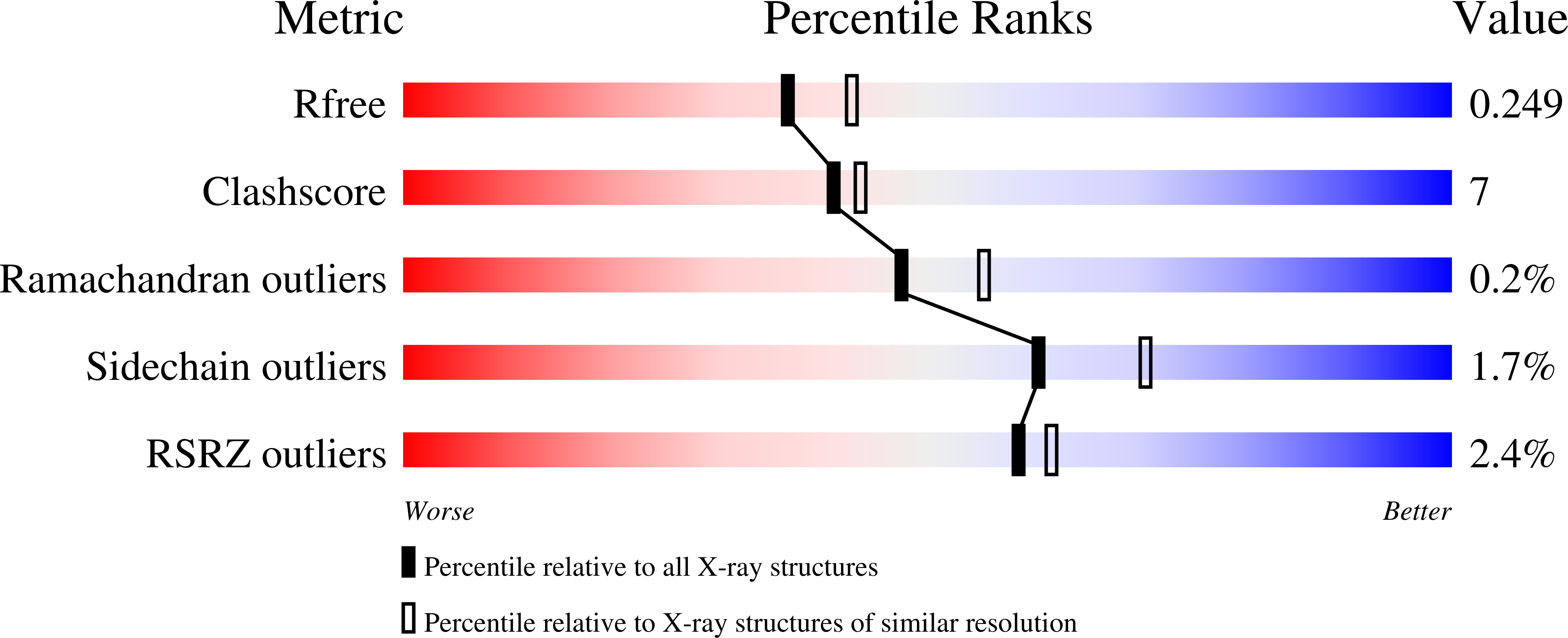
Structure-Function Analyses of a Caffeic Acid O-Methyltransferase from Perennial Ryegrass Reveal the Molecular Basis for Substrate Preference.
Louie, G.V., Bowman, M.E., Tu, Y., Mouradov, A., Spangenberg, G., Noel, J.P.(2010) Plant Cell 22: 4114-4127
- PubMed: 21177481
- DOI: https://doi.org/10.1105/tpc.110.077578
- Primary Citation of Related Structures:
3P9C, 3P9I, 3P9K - PubMed Abstract:
Lignin forms from the polymerization of phenylpropanoid-derived building blocks (the monolignols), whose modification through hydroxylation and O-methylation modulates the chemical and physical properties of the lignin polymer. The enzyme caffeic acid O-methyltransferase (COMT) is central to lignin biosynthesis. It is often targeted in attempts to engineer the lignin composition of transgenic plants for improved forage digestibility, pulping efficiency, or utility in biofuel production. Despite intensive investigation, the structural determinants of the regiospecificity and substrate selectivity of COMT remain poorly defined. Reported here are x-ray crystallographic structures of perennial ryegrass (Lolium perenne) COMT (Lp OMT1) in open conformational state, apo- and holoenzyme forms and, most significantly, in a closed conformational state complexed with the products S-adenosyl-L-homocysteine and sinapaldehyde. The product-bound complex reveals the post-methyl-transfer organization of COMT's catalytic groups with reactant molecules and the fully formed phenolic-ligand binding site. The core scaffold of the phenolic ligand forges a hydrogen-bonding network involving the 4-hydroxy group that anchors the aromatic ring and thereby permits only metahydroxyl groups to be positioned for transmethylation. While distal from the site of transmethylation, the propanoid tail substituent governs the kinetic preference of ryegrass COMT for aldehydes over alcohols and acids due to a single hydrogen bond donor for the C9 oxygenated moiety dictating the preference for an aldehyde.
Organizational Affiliation:
The Howard Hughes Medical Institute, Salk Institute for Biological Studies, La Jolla, California 92037, USA.