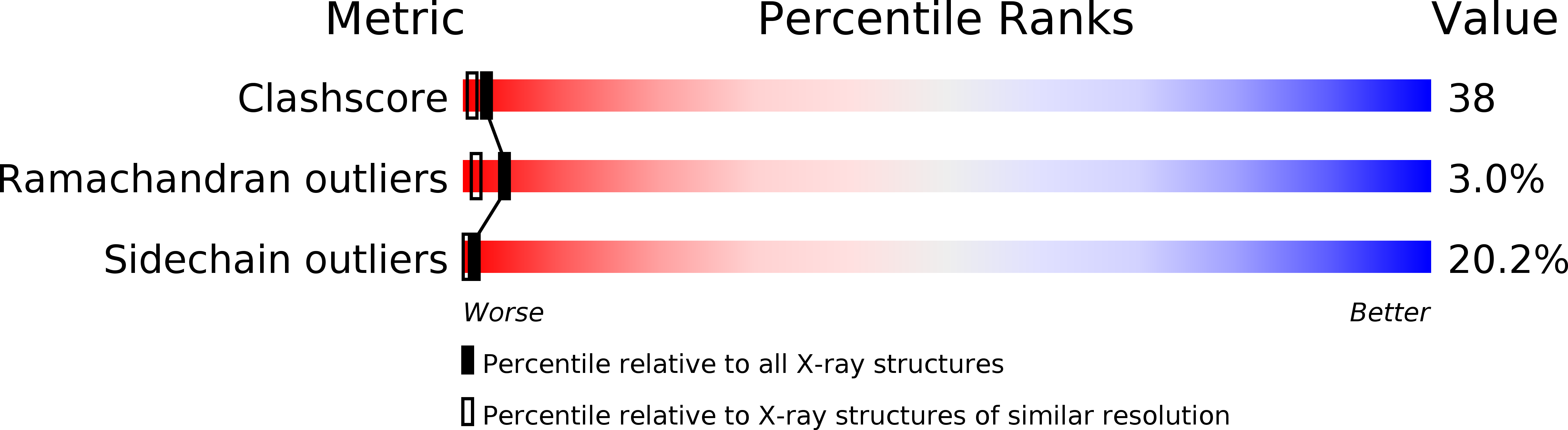Enhanced activity and altered specificity of phospholipase A2 by deletion of a surface loop.
Kuipers, O.P., Thunnissen, M.M., de Geus, P., Dijkstra, B.W., Drenth, J., Verheij, H.M., de Haas, G.H.(1989) Science 244: 82-85
- PubMed: 2704992
- DOI: https://doi.org/10.1126/science.2704992
- Primary Citation of Related Structures:
3P2P - PubMed Abstract:
Protein engineering and x-ray crystallography have been used to study the role of a surface loop that is present in pancreatic phospholipases but is absent in snake venom phospholipases. Removal of residues 62 to 66 from porcine pancreatic phospholipase A2 does not change the binding constant for micelles significantly, but it improves catalytic activity up to 16 times on micellar (zwitterionic) lecithin substrates. In contrast, the decrease in activity on negatively charged substrates is greater than fourfold. A crystallographic study of the mutant enzyme shows that the region of the deletion has a well-defined structure that differs from the structure of the wild-type enzyme. No structural changes in the active site of the enzyme were detected.
Organizational Affiliation:
Department of Biochemistry, University of Utrecht, The Netherlands.