Methylthioinosine phosphorylase from Pseudomonas aeruginosa. Structure and annotation of a novel enzyme in quorum sensing.
Guan, R., Ho, M.C., Almo, S.C., Schramm, V.L.(2011) Biochemistry 50: 1247-1254
- PubMed: 21197954
- DOI: https://doi.org/10.1021/bi101642d
- Primary Citation of Related Structures:
3OZB - PubMed Abstract:
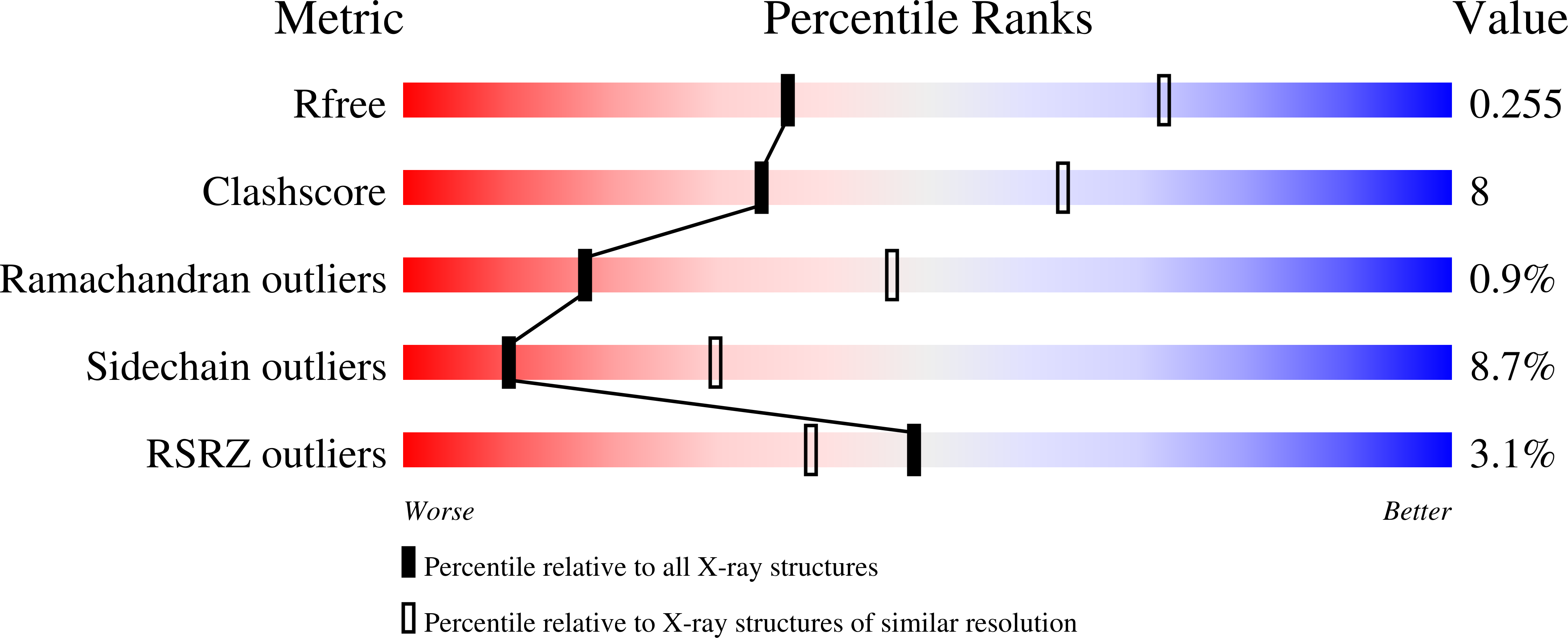
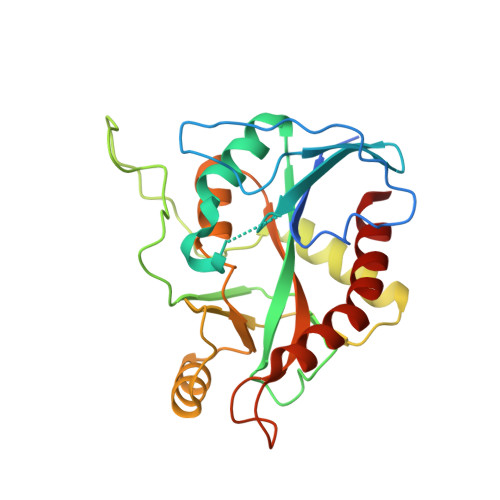
The PA3004 gene of Pseudomonas aeruginosa PAO1 was originally annotated as a 5'-methylthioadenosine phosphorylase (MTAP). However, the PA3004 encoded protein uses 5'-methylthioinosine (MTI) as a preferred substrate and represents the only known example of a specific MTI phosphorylase (MTIP). MTIP does not utilize 5'-methylthioadenosine (MTA). Inosine is a weak substrate with a k(cat)/K(m) value 290-fold less than MTI and is the second best substrate identified. The crystal structure of P. aeruginosa MTIP (PaMTIP) in complex with hypoxanthine was determined to 2.8 Å resolution and revealed a 3-fold symmetric homotrimer. The methylthioribose and phosphate binding regions of PaMTIP are similar to MTAPs, and the purine binding region is similar to that of purine nucleoside phosphorylases (PNPs). The catabolism of MTA in P. aeruginosa involves deamination to MTI and phosphorolysis to hypoxanthine (MTA → MTI → hypoxanthine). This pathway also exists in Plasmodium falciparum, where the purine nucleoside phosphorylase (PfPNP) acts on both inosine and MTI. Three tight-binding transition state analogue inhibitors of PaMTIP are identified with dissociation constants in the picomolar range. Inhibitor specificity suggests an early dissociative transition state for PaMTIP. Quorum sensing molecules are associated with MTA metabolism in bacterial pathogens suggesting PaMTIP as a potential therapeutic target.
Organizational Affiliation:
Department of Biochemistry, Albert Einstein College of Medicine, Yeshiva University, 1300 Morris Park Avenue, Bronx, New York 10461, United States.