Computational reprogramming of homing endonuclease specificity at multiple adjacent base pairs.
Ashworth, J., Taylor, G.K., Havranek, J.J., Quadri, S.A., Stoddard, B.L., Baker, D.(2010) Nucleic Acids Res 38: 5601-5608
- PubMed: 20435674
- DOI: https://doi.org/10.1093/nar/gkq283
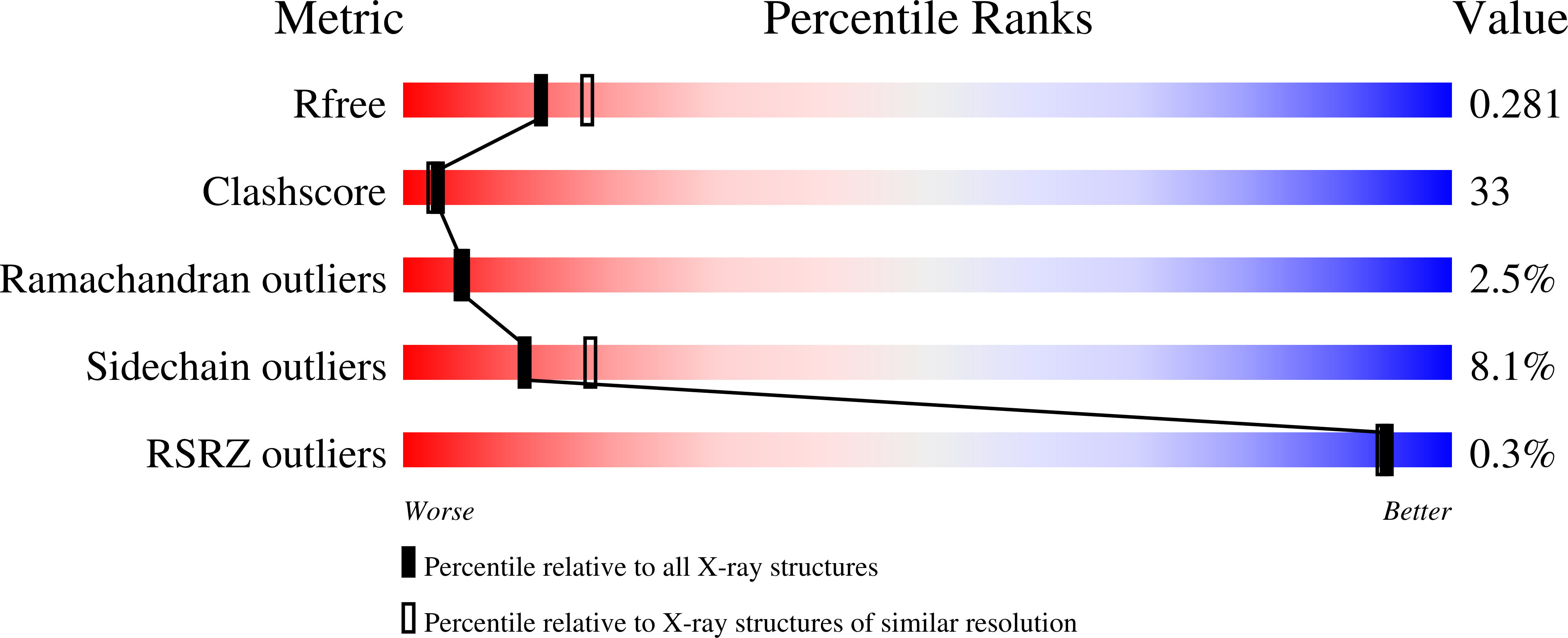
- Primary Citation of Related Structures:
3KO2, 3MIP, 3MIS - PubMed Abstract:
Site-specific homing endonucleases are capable of inducing gene conversion via homologous recombination. Reprogramming their cleavage specificities allows the targeting of specific biological sites for gene correction or conversion. We used computational protein design to alter the cleavage specificity of I-MsoI for three contiguous base pair substitutions, resulting in an endonuclease whose activity and specificity for its new site rival that of wild-type I-MsoI for the original site. Concerted design for all simultaneous substitutions was more successful than a modular approach against individual substitutions, highlighting the importance of context-dependent redesign and optimization of protein-DNA interactions. We then used computational design based on the crystal structure of the designed complex, which revealed significant unanticipated shifts in DNA conformation, to create an endonuclease that specifically cleaves a site with four contiguous base pair substitutions. Our results demonstrate that specificity switches for multiple concerted base pair substitutions can be computationally designed, and that iteration between design and structure determination provides a route to large scale reprogramming of specificity.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195, USA. ashwortj@u.washington.edu