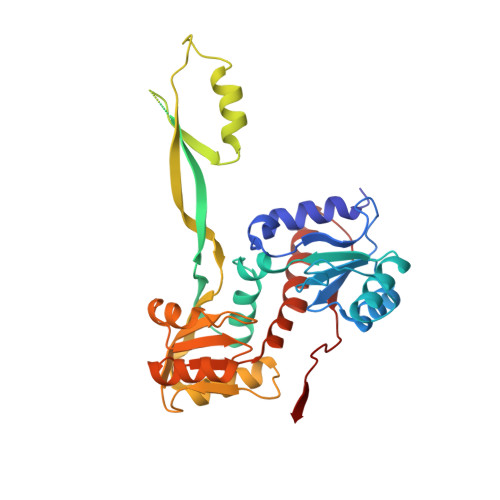
Structure of a Conserved Hypothetical Protein Sa1388 from S. aureus Reveals a Capped Hexameric Toroid with Two Pii Domain Lids and a Dinuclear Metal Center.
Singh Saikatendu, K., Zhang, X., Kinch, L., Leybourne, M., Grishin, N.V., Zhang, H.(2006) BMC Struct Biol 6: 27
- PubMed: 17187687
- DOI: https://doi.org/10.1186/1472-6807-6-27
- Primary Citation of Related Structures:
2NYD, 3LNL - PubMed Abstract:
The protein encoded by the SA1388 gene from Staphylococcus aureus was chosen for structure determination to elucidate its domain organization and confirm our earlier remote homology based prediction that it housed a nitrogen regulatory PII protein-like domain. SA1388 was predicted to contain a central PII-like domain and two flanking regions, which together belong to the NIF3-like protein family. Proteins like SA1388 remain a poorly studied group and their structural characterization could guide future investigations aimed at understanding their function.
Organizational Affiliation:
Department of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas TX 75390-8816, USA. skumar@scripps.edu