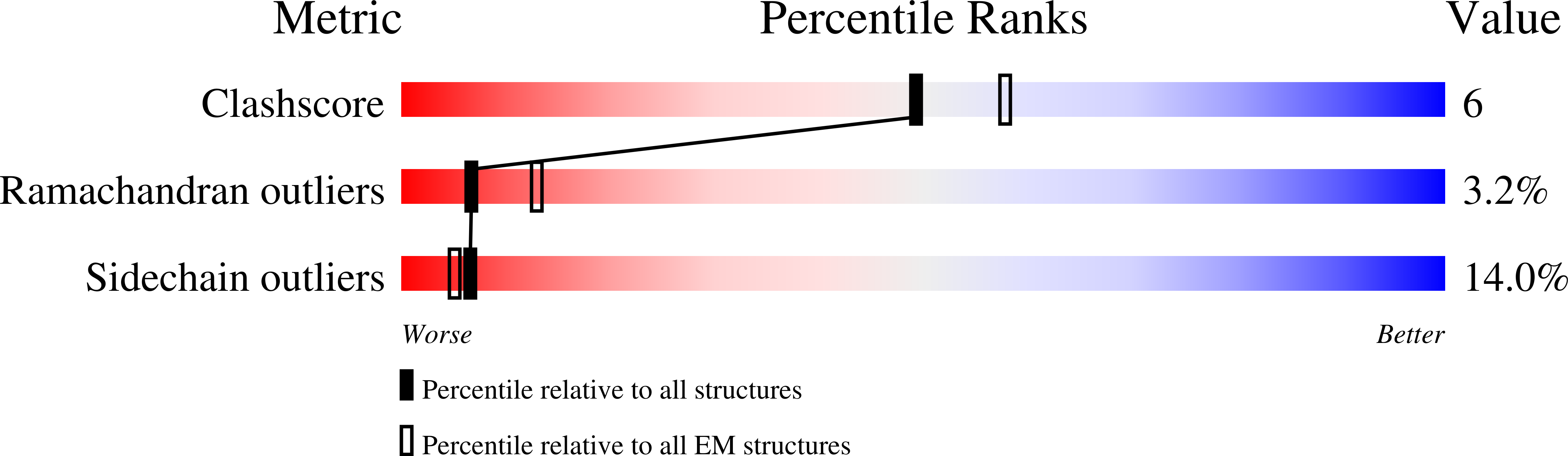Molecular dynamics of EF-G during translocation.
Li, W., Trabuco, L.G., Schulten, K., Frank, J.(2011) Proteins 79: 1478-1486
- PubMed: 21365677
- DOI: https://doi.org/10.1002/prot.22976
- Primary Citation of Related Structures:
3IZP - PubMed Abstract:
Elongation factor G (EF-G) plays a crucial role in two stages of mRNA-(tRNA)(2) translocation. First, EF-G•GTP enters the pre-translocational ribosome in its intersubunit-rotated state, with tRNAs in their hybrid (P/E and A/P) positions. Second, a conformational change in EF-G's Domain IV induced by GTP hydrolysis disengages the mRNA-anticodon stem-loops of the tRNAs from the decoding center to advance relative to the small subunit when the ribosome undergoes a backward inter-subunit rotation. These events take place as EF-G undergoes a series of large conformational changes as visualized by cryo-EM and X-ray studies. The number and variety of these structures leave open many questions on how these different configurations form during the dynamic translocation process. To understand the molecular mechanism of translocation, we examined the molecular motions of EF-G in solution by means of molecular dynamics simulations. Our results show: (1) rotations of the super-domain formed by Domains III-V with respect to the super-domain formed by I-II, and rotations of Domain IV with respect to Domain III; (2) flexible conformations of both 503- and 575-loops; (3) large conformational variability in the bound form caused by the interaction between Domain V and the GTPase-associated center; (4) after GTP hydrolysis, the Switch I region seems to be instrumental for effecting the conformational change at the end of Domain IV implicated in the disengagement of the codon-anticodon helix from the decoding center.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, New York, New York 10032, USA.