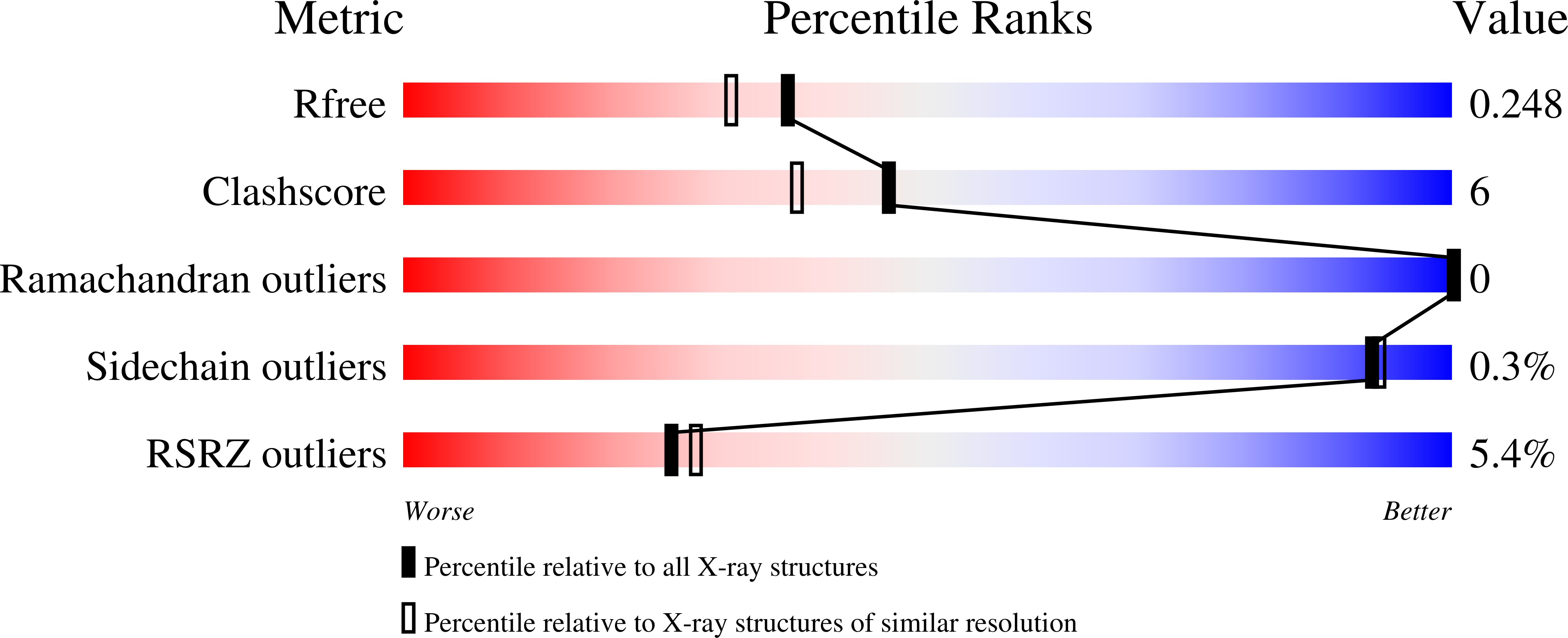
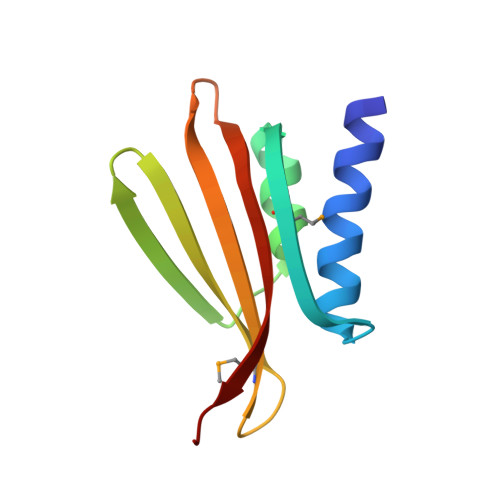
The thermo- and acido-stable ORF-99 from the archaeal virus AFV1
Goulet, A., Spinelli, S., Blangy, S., van Tilbeurgh, H., Leulliot, N., Basta, T., Prangishvili, D., Cambillau, C., Campanacci, V.(2009) Protein Sci 18: 1316-1320
- PubMed: 19472363
- DOI: https://doi.org/10.1002/pro.122
- Primary Citation of Related Structures:
3DF6, 3DJW - PubMed Abstract:
Acidianus Filamentous Virus 1 (AFV1), isolated from acidic hot springs, is an enveloped lipid-containing archaeal filamentous virus with a linear double-stranded DNA genome. It infects Acidianus, which is a hyperthermostable archaea growing at 85 degrees C and acidic pHs, below pH 3. AFV1-99, a protein of 99 amino acids of unknown function, has homologues in the archaeal virus families Lipothrixviridae and Rudiviridae. We determined the crystal structure of AFV1-99 at 2.05 A resolution. AFV1-99 has a new fold, is hyperthermostable (up to 95 degrees C) and resists to extreme pH (between pH 0 and 11) and to the combination of high temperature (95 degrees C) and low pH (pH 0). It possesses characteristics of hyperthermostable proteins, such as a high content of charged residues.
Organizational Affiliation:
Architecture et Fonction des Macromolécules Biologiques, CNRS and Universités d'Aix-Marseille I & II, 163 avenue de Luminy, Marseille cedex 9, France.