Two distinct mechanisms for the regulation of actin capping protein-competitive or allosteric inhibitions
Takeda, S., Minakata, S., Narita, A., Kitazawa, M., Yamakuni, T., Maeda, Y., Nitanai, Y.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| F-actin-capping protein subunit alpha-1 | 286 | Gallus gallus | Mutation(s): 0 Gene Names: CAPZA1 | 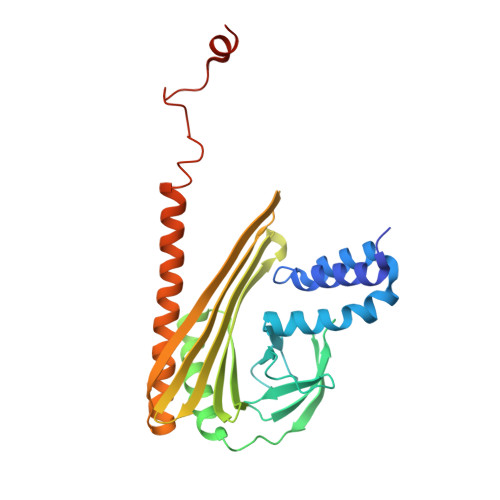 | |
UniProt | |||||
Find proteins for P13127 (Gallus gallus) Explore P13127 Go to UniProtKB: P13127 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P13127 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| F-actin-capping protein subunit beta isoforms 1 and 2 | 277 | Gallus gallus | Mutation(s): 0 Gene Names: CAPZB | 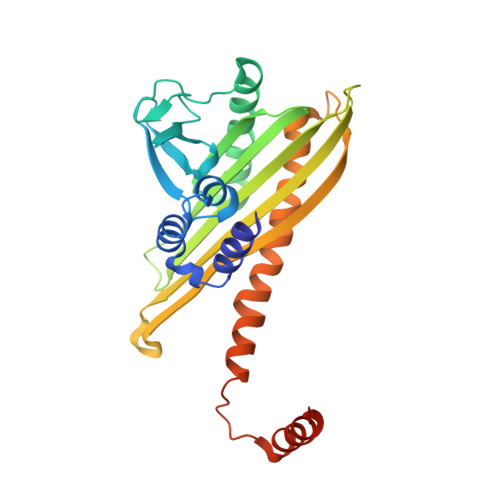 | |
UniProt | |||||
Find proteins for P14315 (Gallus gallus) Explore P14315 Go to UniProtKB: P14315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P14315 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 32mer peptide from Leucine-rich repeat-containing protein 16A | K [auth X], L [auth Y], M [auth Z], N [auth W], O [auth V] | 37 | Mus musculus | Mutation(s): 0 Gene Names: Lrrc16a | 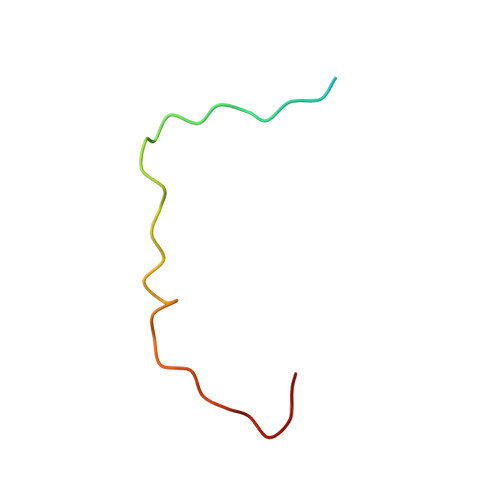 |
UniProt | |||||
Find proteins for Q6EDY6 (Mus musculus) Explore Q6EDY6 Go to UniProtKB: Q6EDY6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q6EDY6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 231.153 | α = 90 |
| b = 104.358 | β = 119.09 |
| c = 186.633 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| MOLREP | phasing |
| REFMAC | refinement |
| DPS | data reduction |
| HKL-2000 | data scaling |