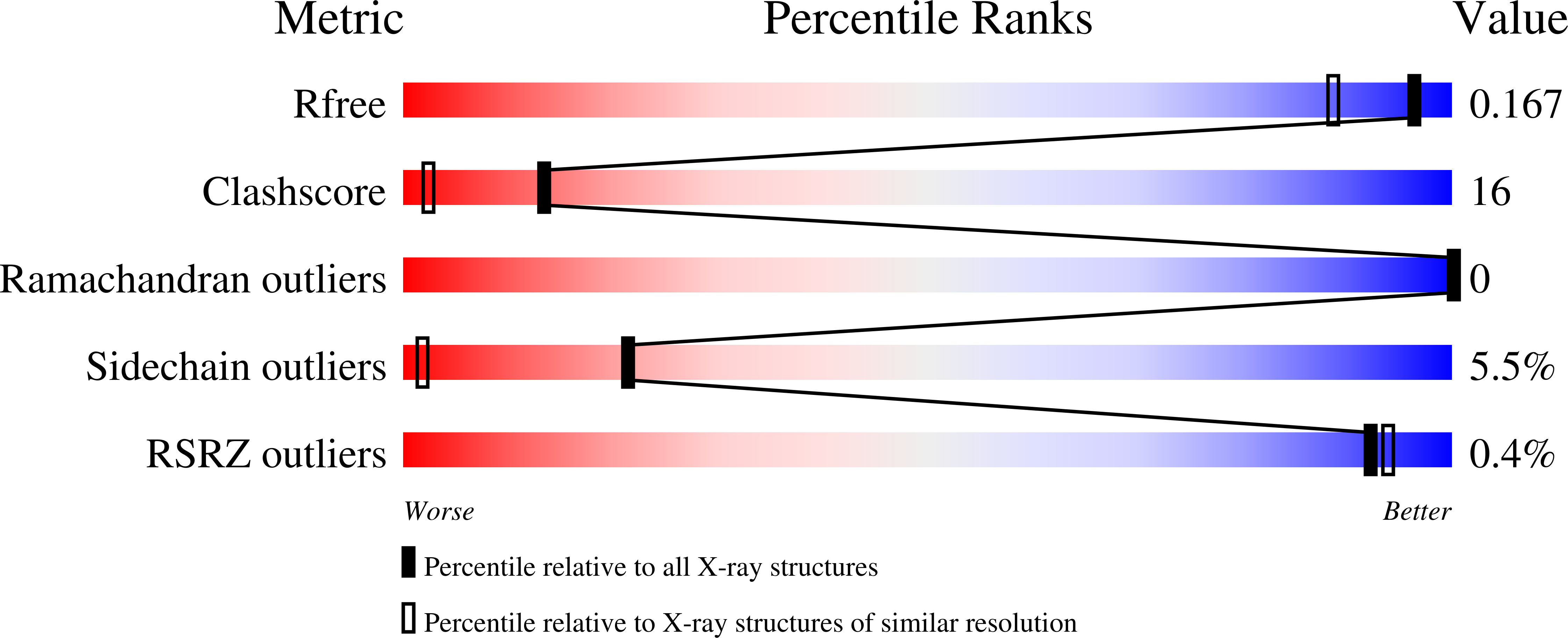
Crystal structure of family 14 polysaccharide lyase with pH-dependent modes of action
Ogura, K., Yamasaki, M., Yamada, T., Mikami, B., Hashimoto, W., Murata, K.(2009) J Biol Chem 284: 35572-35579
- PubMed: 19846561
- DOI: https://doi.org/10.1074/jbc.M109.068056
- Primary Citation of Related Structures:
3A0N, 3GNE, 3IM0 - PubMed Abstract:
The Chlorella virus enzyme vAL-1 (38 kDa), a member of polysaccharide lyase family 14, degrades the Chlorella cell wall by cleaving the glycoside bond of the glucuronate residue (GlcA) through a beta-elimination reaction. The enzyme consists of an N-terminal cell wall-attaching domain (11 kDa) and a C-terminal catalytic module (27 kDa). Here, we show the enzyme characteristics of vAL-1, especially its pH-dependent modes of action, and determine the structure of the catalytic module. vAL-1 also exhibited alginate lyase activity at alkaline pH, and truncation of the N-terminal domain increased the lyase activity by 50-fold at pH 7.0. The truncated form vAL-1(S) released di- to hexasaccharides from alginate at pH 7.0, whereas disaccharides were preferentially generated at pH 10.0. This indicates that vAL-1(S) shows two pH-dependent modes of action: endo- and exotypes. The x-ray crystal structure of vAL-1(S) at 1.2 A resolution showed two antiparallel beta-sheets with a deep cleft showing a beta-jelly roll fold. The structure of GlcA-bound vAL-1(S) at pH 7.0 and 10.0 was determined: GlcA was found to be bound outside and inside the cleft at pH 7.0 and 10.0, respectively. This suggests that the electric charges at the active site greatly influence the binding mode of substrates and regulate endo/exo activity. Site-directed mutagenesis demonstrated that vAL-1(S) has a specific amino acid arrangement distinct from other alginate lyases crucial for catalysis. This is, to our knowledge, the first study in which the structure of a family 14 polysaccharide lyase with two different modes of action has been determined.
Organizational Affiliation:
Division of Food Science and Biotechnology, Graduate School of Agriculture, Kyoto University, Gokasho, Uji 611-0011 , Japan.