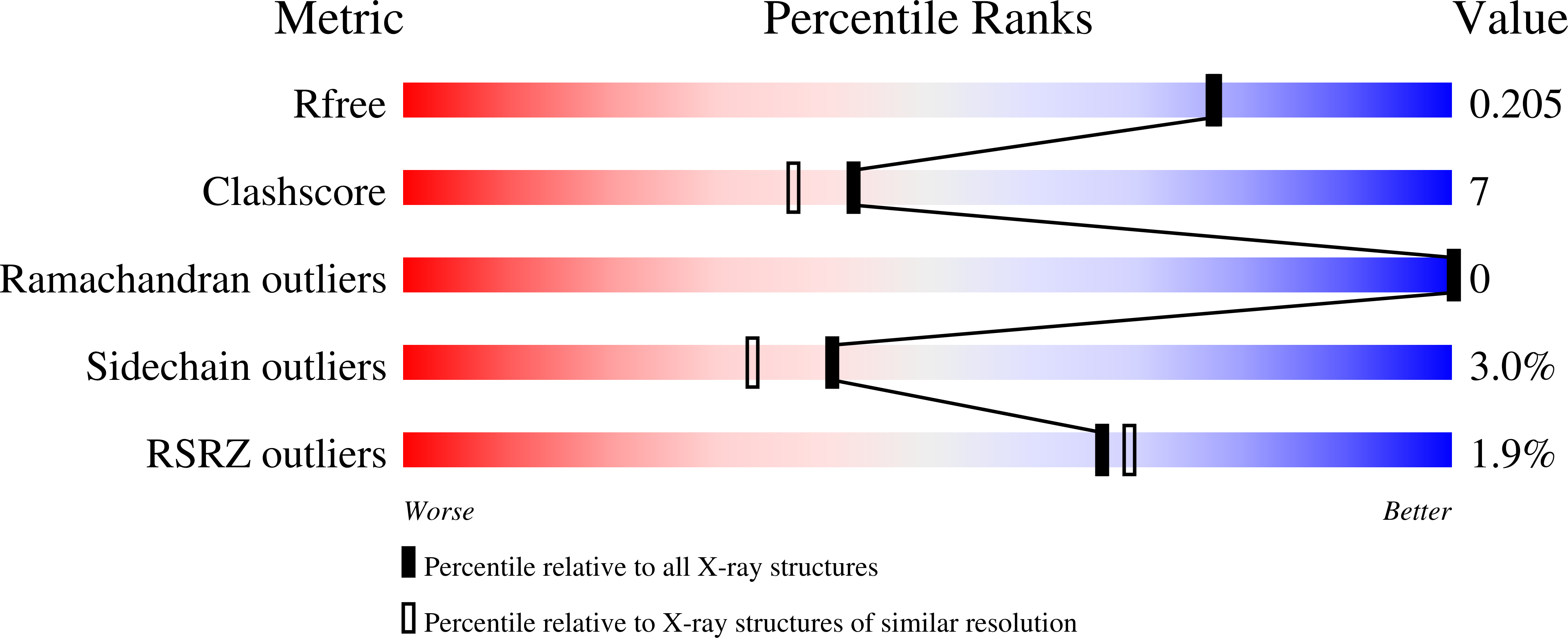
GES-18, a new carbapenem-hydrolyzing GES-Type beta-lactamase from pseudomonas aeruginosa that contains Ile80 and Ser170 residues.
Bebrone, C., Bogaerts, P., Delbruck, H., Bennink, S., Kupper, M.B., Rezende de Castro, R., Glupczynski, Y., Hoffmann, K.M.(2013) Antimicrob Agents Chemother 57: 396-401
- PubMed: 23114760
- DOI: https://doi.org/10.1128/AAC.01784-12
- Primary Citation of Related Structures:
3V3S - PubMed Abstract:
A clinical isolate of Pseudomonas aeruginosa recovered from the lower respiratory tract of an 81-year-old patient hospitalized in Belgium was sent to the national reference center to determine its resistance mechanism. PCR sequencing identified a new GES variant, GES-18, which differs from the carbapenem-hydrolyzing enzyme GES-5 by a single amino acid substitution (Val80Ile, in the numbering according to Ambler) and from GES-1 by two substitutions (Val80Ile and Gly170Ser). Detailed kinetic characterization showed that GES-18 and GES-5 hydrolyze imipenem and cefoxitin with similar kinetic parameters and that GES-18 was less susceptible than GES-1 to classical β-lactamase inhibitors such as clavulanate and tazobactam. The overall structure of GES-18 is similar to the solved structures of GES-1 and GES-2, the Val80Ile and Gly170Ser substitutions causing only subtle local rearrangements. Notably, the hydrolytic water molecule and the Glu166 residue were slightly displaced compared to their counterparts in GES-1. Our kinetic and crystallographic data for GES-18 highlight the pivotal role of the Gly170Ser substitution which distinguishes GES-5 and GES-18 from GES-1.
Organizational Affiliation:
Institute of Molecular Biotechnology, RWTH-Aachen University, c/o Fraunhofer IME, Aachen, Germany. carine.bebrone@molbiotech