Structural origins of DNA target selection and nucleobase extrusion by a DNA Cytosine methyltransferase.
Didovyk, A., Verdine, G.L.(2012) J Biol Chem 287: 40099-40105
- PubMed: 23012373
- DOI: https://doi.org/10.1074/jbc.M112.413054
- Primary Citation of Related Structures:
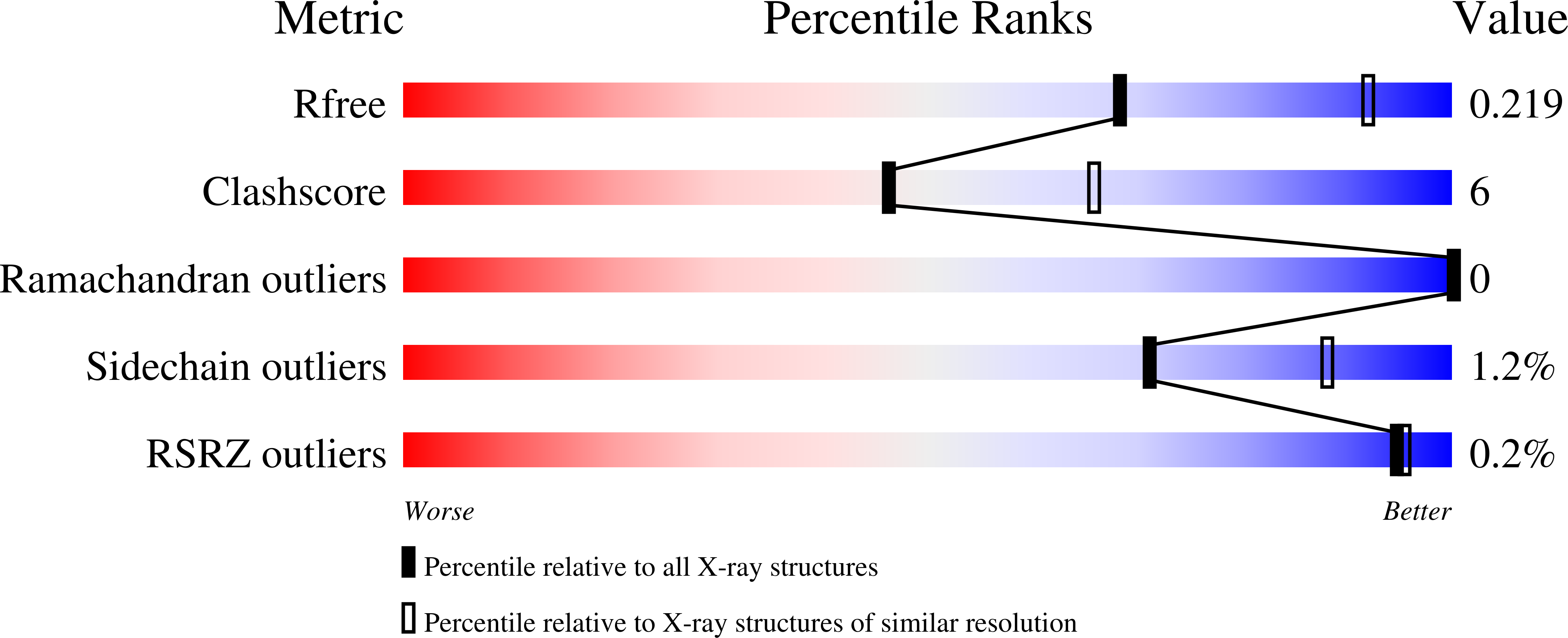
3UBT - PubMed Abstract:
How DNA 5-cytosine methyltransferases (DCMTases) select their substrate nucleobase for extrusion from DNA duplex is poorly understood. The crystal structure of a pre-extrusion M.HaeIII DCMTase-substrate DNA complex is reported here. M.HaeIII selects its substrate cytosine for extrusion by selectively interfering with its stacking and hydrogen bonding interactions within the DNA duplex. This is the first structural elucidation of the target cytosine selection by a DCMTase. Epigenetic methylation of cytosine residues in DNA is an essential element of genome maintenance and function in organisms ranging from bacteria to humans. DNA 5-cytosine methyltransferase enzymes (DCMTases) catalyze cytosine methylation via reaction intermediates in which the DNA is drastically remodeled, with the target cytosine residue extruded from the DNA helix and plunged into the active site pocket of the enzyme. We have determined a crystal structure of M.HaeIII DCMTase in complex with its DNA substrate at a previously unobserved state, prior to extrusion of the target cytosine and frameshifting of the DNA recognition sequence. The structure reveals that M.HaeIII selects the target cytosine and destabilizes its base-pairing through a precise, focused, and coordinated assault on the duplex DNA, which isolates the target cytosine from its nearest neighbors and thereby facilitates its extrusion from DNA.
Organizational Affiliation:
Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138, USA.