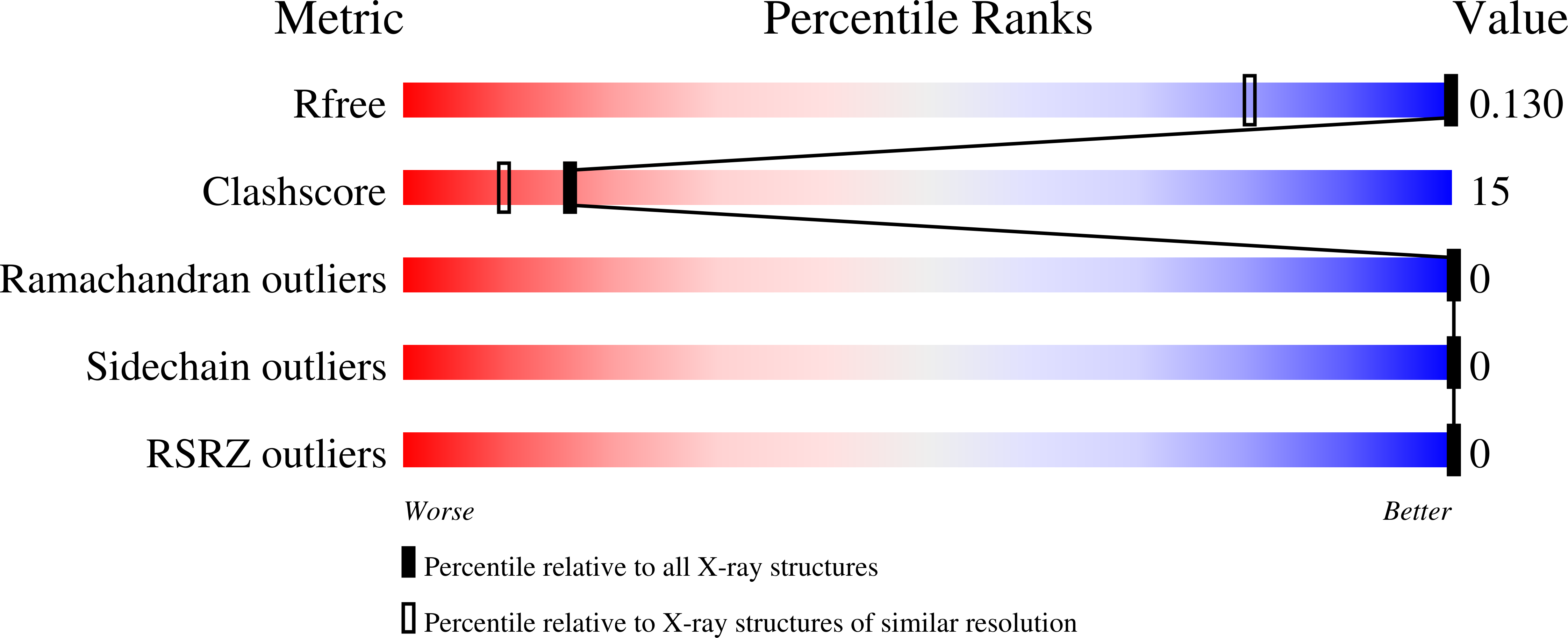
Four complete turns of a curved 310-helix at atomic resolution: The crystal structure of the peptaibol trichovirin I-4A in polar environment suggests a transition to alpha-helix for membrane function
Gessmann, R., Axford, D., Owen, R.L., Bruckner, H., Petratos, K.(2012) Acta Crystallogr D Biol Crystallogr 68: 109-116
- PubMed: 22281739
- DOI: https://doi.org/10.1107/S090744491105133X
- Primary Citation of Related Structures:
3SBN - PubMed Abstract:
The first crystal structure of a member of peptaibol antibiotic subfamily 4, trichovirin I-4A (14 residues), has been determined by direct methods and refined at atomic resolution. The monoclinic unit cell has two molecules in the asymmetric unit. Both molecules assume a 3₁₀ right-handed helical conformation and are significantly bent. The molecules pack loosely along the crystallographic twofold axis, forming two large tunnels between symmetry-related molecules in which no ordered solvent could be located. Carbonyl O atoms which are not involved in intramolecular hydrogen bonding participate in close van der Waals interactions with apolar groups. The necessary amphipathicity for biological activity of peptaibols is not realised in the crystal structure. Hence, a structural change of trichovirin to an α-helical conformation is proposed for membrane integration and efficient water/ion transportation across the lipid bilayer.
Organizational Affiliation:
IMBB-FORTH, N. Plastira 100, Heraklion, Crete 70013, Greece.