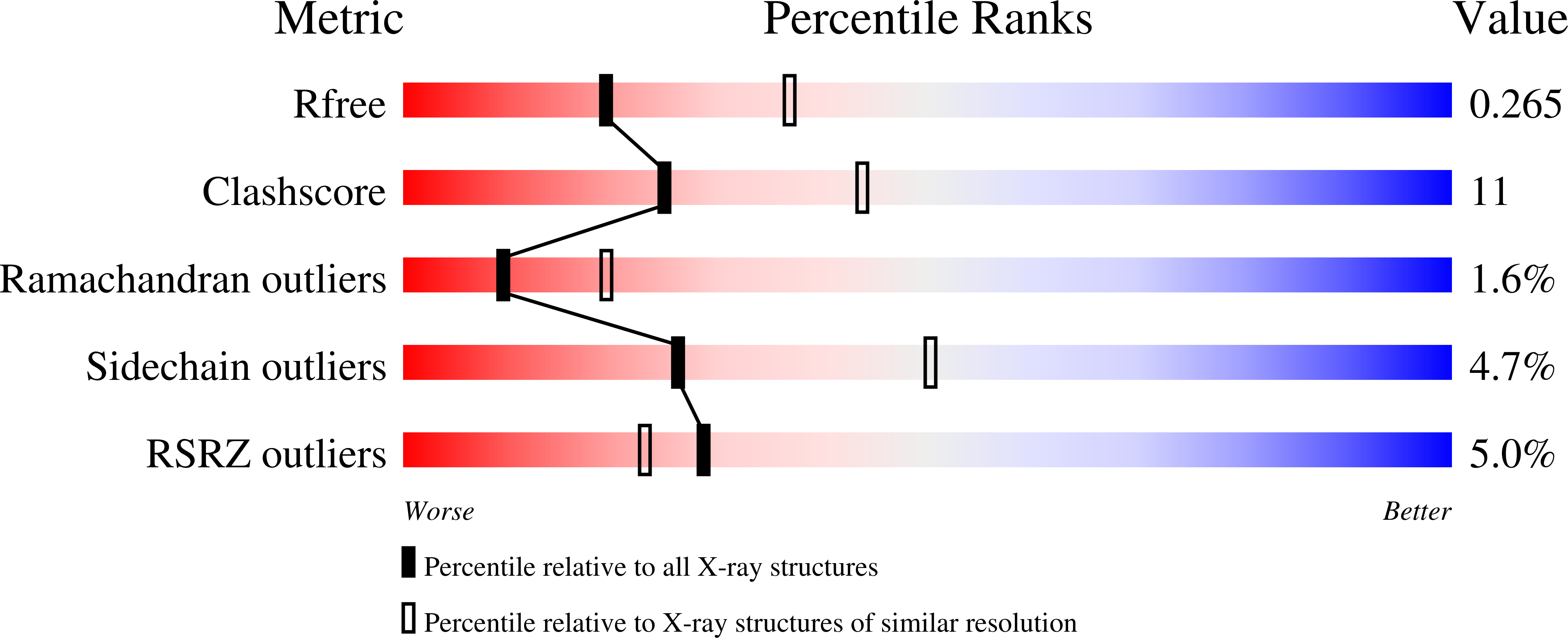
Fragment-based discovery and optimization of BACE1 inhibitors.
Madden, J., Dod, J.R., Godemann, R., Kraemer, J., Smith, M., Biniszkiewicz, M., Hallett, D.J., Barker, J., Dyekjaer, J.D., Hesterkamp, T.(2010) Bioorg Med Chem Lett 20: 5329-5333
- PubMed: 20656487
- DOI: https://doi.org/10.1016/j.bmcl.2010.06.089
- Primary Citation of Related Structures:
3MSJ, 3MSK, 3MSL, 3S2O - PubMed Abstract:
A novel series of 2-aminobenzimidazole inhibitors of BACE1 has been discovered using fragment-based drug discovery (FBDD) techniques. The rapid optimization of these inhibitors using structure-guided medicinal chemistry is discussed.
Organizational Affiliation:
Evotec UK Ltd, Abingdon, UK. james.madden@evotec.com