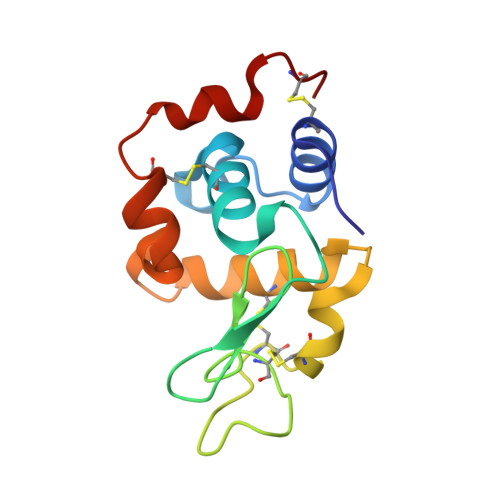
Post-protein-binding reactivity and modifications of the fac-[Re(CO)3]+ core
Zobi, F., Spingler, B.(2012) Inorg Chem 51: 1210-1212
- PubMed: 22229733
- DOI: https://doi.org/10.1021/ic2023314
- Primary Citation of Related Structures:
3QE8, 3QNG - PubMed Abstract:
The reactivity of the [Re(CO)(3)(H(2)O)(2)](+) complex coordinated to the His15 residue of HEW lysozyme is described. In the fully metalated protein (Lys-1), the Re ion retains its reactivity only toward selected ligands, while others induce a ligand-mediated demetalation of the enzyme. It is further shown that some of the complexes that may be "engineered" on the lysozyme do not react with the free protein even if present in solution in excess. The formation of stable metal adducts starting from Lys-1 was confirmed by X-ray crystallography.
Organizational Affiliation:
Institute of Inorganic Chemistry, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland. fzobi@aci.uzh.ch