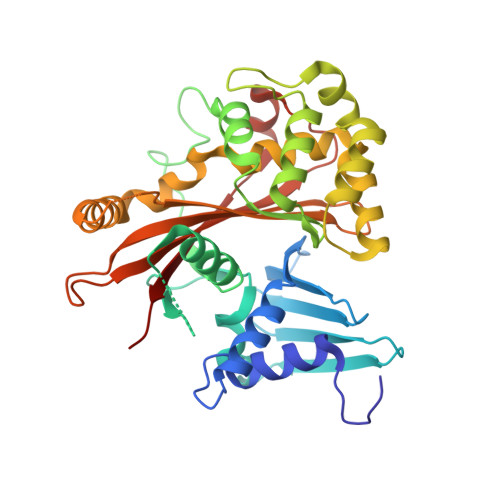
The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold.
Selyunin, A.S., Sutton, S.E., Weigele, B.A., Reddick, L.E., Orchard, R.C., Bresson, S.M., Tomchick, D.R., Alto, N.M.(2011) Nature 469: 107-111
- PubMed: 21170023
- DOI: https://doi.org/10.1038/nature09593
- Primary Citation of Related Structures:
3PCR, 3PCS - PubMed Abstract:
The fidelity and specificity of information flow within a cell is controlled by scaffolding proteins that assemble and link enzymes into signalling circuits. These circuits can be inhibited by bacterial effector proteins that post-translationally modify individual pathway components. However, there is emerging evidence that pathogens directly organize higher-order signalling networks through enzyme scaffolding, and the identity of the effectors and their mechanisms of action are poorly understood. Here we identify the enterohaemorrhagic Escherichia coli O157:H7 type III effector EspG as a regulator of endomembrane trafficking using a functional screen, and report ADP-ribosylation factor (ARF) GTPases and p21-activated kinases (PAKs) as its relevant host substrates. The 2.5 Å crystal structure of EspG in complex with ARF6 shows how EspG blocks GTPase-activating-protein-assisted GTP hydrolysis, revealing a potent mechanism of GTPase signalling inhibition at organelle membranes. In addition, the 2.8 Å crystal structure of EspG in complex with the autoinhibitory Iα3-helix of PAK2 defines a previously unknown catalytic site in EspG and provides an allosteric mechanism of kinase activation by a bacterial effector. Unexpectedly, ARF and PAKs are organized on adjacent surfaces of EspG, indicating its role as a 'catalytic scaffold' that effectively reprograms cellular events through the functional assembly of GTPase-kinase signalling complex.
Organizational Affiliation:
Department of Microbiology, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas 75390-8816, USA.