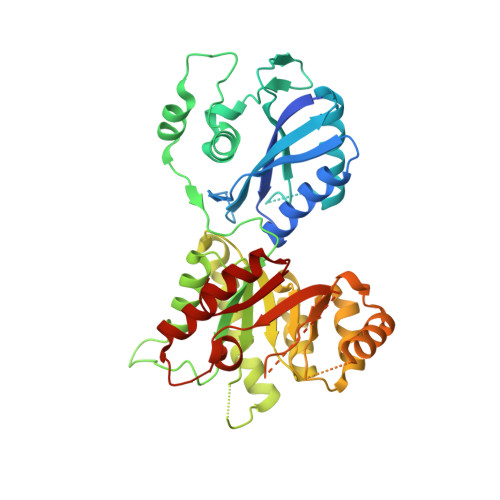
Crystal structure of the 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase.dihydropteroate synthase bifunctional enzyme from Francisella tularensis.
Pemble, C.W., Mehta, P.K., Mehra, S., Li, Z., Nourse, A., Lee, R.E., White, S.W.(2010) PLoS One 5: e14165-e14165
- PubMed: 21152407
- DOI: https://doi.org/10.1371/journal.pone.0014165
- Primary Citation of Related Structures:
3MCM, 3MCN, 3MCO - PubMed Abstract:
The 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase (HPPK) and dihydropteroate synthase (DHPS) enzymes catalyze sequential metabolic reactions in the folate biosynthetic pathway of bacteria and lower eukaryotes. Both enzymes represent validated targets for the development of novel anti-microbial therapies. We report herein that the genes which encode FtHPPK and FtDHPS from the biowarfare agent Francisella tularensis are fused into a single polypeptide. The potential of simultaneously targeting both modules with pterin binding inhibitors prompted us to characterize the molecular details of the multifunctional complex. Our high resolution crystallographic analyses reveal the structural organization between FtHPPK and FtDHPS which are tethered together by a short linker. Additional structural analyses of substrate complexes reveal that the active sites of each module are virtually indistinguishable from those of the monofunctional enzymes. The fused bifunctional enzyme therefore represents an excellent vehicle for finding inhibitors that engage the pterin binding pockets of both modules that have entirely different architectures. To demonstrate that this approach has the potential of producing novel two-hit inhibitors of the folate pathway, we identify and structurally characterize a fragment-like molecule that simultaneously engages both active sites. Our study provides a molecular framework to study the enzyme mechanisms of HPPK and DHPS, and to design novel and much needed therapeutic compounds to treat infectious diseases.
Organizational Affiliation:
Department of Structural Biology, St. Jude Children's Research Hospital, and Department of Molecular Sciences, University of Tennessee Health Science Center, Memphis, Tennessee, United States of America.